Abstract
Nitric oxide (NO) is an important plant signaling molecule that has a vital role in abiotic stress tolerance. In the present study, we assessed drought-induced (15 and 30% PEG, polyethylene glycol) damage in wheat (Triticum aestivum L. cv. Prodip) seedlings and mitigation by the synergistic effect of exogenous Arg (0.5 mM l-Arginine) and an NO donor (0.5 mM sodium nitroprusside, SNP). Drought stress sharply decreased the leaf relative water content (RWC) but markedly increased the proline (Pro) content in wheat seedlings. Drought stress caused overproduction of reactive oxygen species (ROS) and methylglyoxal (MG) due to the inefficiency of antioxidant enzymes, the glyoxalase system, and the ascorbate-glutathione pool. However, supplementation with the NO donor and Arg enhanced the antioxidant defense system (both non-enzymatic and enzymatic components) in drought-stressed seedlings. Application of the NO donor and Arg also enhanced the glyoxalase system and reduced the MG content by increasing the activities of the glyoxalase system enzymes (Gly I and Gly II), which restored the leaf RWC and further increased the Pro content under drought stress conditions. Exogenous NO donor and Arg application enhanced the endogenous NO content, which positively regulated the antioxidant system and reduced ROS production. Thus, the present study reveals the crucial roles of Arg and NO in enhancing drought stress tolerance in wheat seedlings by upgrading their water status and reducing oxidative stress and MG toxicity.
Keywords: Amino acid, AsA–GSH pathway, Glutathione, Osmotic stress, Oxidative stress, Phytohormone
Introduction
Drought stress is responsible for water scarcity in plants, the primary effect of which is a decrease in the tissue water content (Kirkham 2005). Such a decrease in water content severely hampers growth, water uptake, nutrient uptake and mobilization, photosynthesis, transpiration, and assimilate translocation (Kocheva et al. 2009; Belkheiri and Mulas 2013; Hasanuzzaman et al. 2014). Oxidative stress is a primary outcome of drought, and overproduction of reactive oxygen species (ROS), such as superoxide anion (O·−2), hydroxyl radical (·OH), hydrogen peroxide (H2O2) and singlet oxygen (1O2), cause photoinhibition and oxidative damage to lipids, proteins, and nucleic acids and disrupts enzyme activity, leading to cell death (Gill and Tuteja 2010; Hasanuzzaman et al. 2012). The antioxidant defense system of plants scavenges ROS to maintain a state of balance under non-stress conditions and involves antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), glutathione peroxidase (GPX), and glutathione S-transferase (GST). These enzymes function in tandem with certain non-enzymatic antioxidants such as ascorbate (AsA), glutathione (GSH), and α-tocopherols to scavenge ROS and alleviate oxidative stress (Hasanuzzaman et al. 2012).
Production of methylglyoxal (MG) is another spontaneous and inevitable by product of the glycolysis pathway; MG is overproduced under environmental stress and has toxic effects on cell components. The glyoxalase system scavenges MG to maintain a balance (Yadav et al. 2005). Utilizing GSH, glyoxalase I (Gly I) converts MG to S-d-lactoylglutathione, and glyoxalase II (Gly II) converts S-d-lactoylglutathione to d-lactic acid. Detoxification of both ROS and MG is considered a strategy for stress tolerance in plants (Yadav et al. 2008; Hasanuzzaman et al. 2012; Rahman et al. 2016a, b).
Nitric oxide (NO) is a highly diffusible gaseous reactive free radical that can permeate through cell membranes. By enhancing expression of antioxidant genes (Hao and Zhang 2010) and activating antioxidant enzymes (Hao and Zhang 2010; Hasanuzzaman et al. 2010; 2018a, b), NO directly enhances ROS scavenging to avoid oxidative damage. NO is also considered to act as a phytohormone, serving as a signal in hormonal and defense responses such as stomatal closure, root development, apoptosis and stress responses (Freschi 2013; Oz et al. 2015). NO regulates both the life processes of plants as well as environmental stress tolerance. In several plant species, NO regulates programmed cell death (PCD), salinity, drought, high and low-temperature stresses, and metal toxicity-induced responses (Wendehenne et al. 2005). Overall, NO has a signaling function and is a potent antioxidant, whereby the signaling function of NO activates redox- and defense-related gene expression in the stress signaling and tolerance cascade (Polverari et al. 2003; Sung and Hong 2010). Previous studies have demonstrated that exogenous NO application confers stress tolerance by enhancing antioxidant defense mechanisms (Neill et al. 2002a, b; Wang and Yang 2005; Sheokand et al. 2008; Zheng et al. 2009; Singh et al. 2009; Xu et al. 2010; Hasanuzzaman et al. 2010; 2018a, b).
Arginine (Arg) is the immediate precursor of NO, urea, amino acid, ornithine, and agmatine (Winter et al. 2015) and is also the precursor of creatine, polyamines (PAs), and glutamate (Tapiero et al. 2002). Thus, Arg might play crucial roles in stress recovery, as it is the most versatile amino acid linked to the biosynthesis of signaling molecules. Arginine has important roles in nitrogen metabolism in germinating seeds and in developing seedlings (Todd et al. 2001), and the roles of Arg have been demonstrated in both animals and fungi (Freedland et al. 1984; Yu and Weiss 1992). However, only a few reports to date highlight its mechanisms and role in development and stress tolerance in plants. Because Arg is a source of enzymatic NO synthesis, we speculated that it may have a function similar to that of NO. Moreover, exogenous Arg-induced regulation of endogenous NO and proline (Pro) levels and their coordinated action in drought stress tolerance is yet to be explored. Therefore, we examined the effects of an NO donor and Arg in improving the defense system and other physiological parameters of wheat plants under conditions of drought stress.
Materials and methods
Plant species and experimental conditions
Wheat (Triticum aestivum L. cv. Pradip) seeds were sterilized for 10 min with 70% ethanol and thoroughly washed with distilled water. The seeds were then imbibed with distilled water for 10 min and germinated on six layers of filter paper in Petri plates (9 cm) in a dark germination chamber for 48 h. Germinated seedlings were transferred to a growth chamber (300 µmol photons m−2 s−1, 25 ± 2 °C and relative humidity of 65–70%) after three days. Hyponex (Hyponex, Japan) diluted 10,000-fold was provided as a source of nutrients. Seven-day-old wheat seedlings at the 4-leaf stage (from sowing) were treated with 0.5 mM sodium nitroprusside (Wako, Japan). SNP was used as an NO donor, and Arg (0.5 mM l-Arginine) was applied alone and as co-treatment with two different levels of drought stress. In this experiment, 15 and 30%, of polyethylene glycol (PEG) solution were considered as mild and severe drought stresses, respectively. Plants grown with the Hyponex solution were used as control plants. Fresh leaves from 9-day-old seedlings were sampled to assess various physiological and biochemical parameters after two days of treatment.
Determination of chl content
For chlorophyll (chl) content determination, fresh leaves were homogenized in 20 volumes of acetone (80% w/v), followed by centrifugation at 5000×g for 10 min. Absorbance was measured with a UV–visible spectrophotometer at 663 and 645 nm and chl contents were calculated using the equations proposed by Arnon (1949).
Estimation of proline content
Proline levels in leaves were determined according to the method of Bates et al. (1973). After homogenization with 3% sulphosalicylic acid, the supernatant was added to ninhydrin with glacial acetic acid and phosphoric acid. After subsequent boiling and cooling, toluene was added, and the sample was vortexed thoroughly. The upper chromophore layer bearing toluene was assessed at 520 nm using a UV–visible spectrophotometer, and the Pro content was calculated against a standard curve.
Determination of the leaf relative water content (RWC)
The fresh weight (FW) of leaves was recorded. The turgid weight (TW) was recorded when leaves became turgid after being dipped in water, and the dry weight (DW) of leaves was measured after oven drying. RWC was calculated using the following formula: RWC (%) = [(FW − DW)/(TW − DW)] × 100 (Barrs and Weatherley 1962).
Measurement of lipid peroxidation, H2O2 content and O·−2 generation rate
Malondialdehyde (MDA), a product of lipid peroxidation, was assessed as an indicator of oxidative damage (Nahar et al. 2016a). To determine H2O2 levels, leaves were homogenized in potassium phosphate buffer (pH 6.5) and centrifuged at 11,500×g. The supernatant was mixed with a reaction mixture (TiCl4 in 20% H2SO4, v/v); after 10 min, the mixture was centrifuged, and absorbance was measured at 410 nm using a UV–visible spectrophotometer (Yu et al. 2003). The generation rate of O·−2 was detected by homogenizing leaves in K–P buffer followed by centrifugation at 5000×g. After 10 min, extraction buffer and the hydroxylamine hydrochloride were added to the supernatant for incubation, mixed with sulfanilamide and naphthylamine and then assessed at 530 nm using a UV–visible spectrophotometer (Hasanuzzaman et al. 2011; Nahar et al. 2016a).
Detection of hydrogen peroxide and superoxide contents
H2O2 and O·−2 were detected through histochemical staining of leaves with 1% 3,3-diaminobenzidine (DAB) and 0.1% nitroblue tetrazolium chloride (NBT) solutions, respectively (Chen et al. 2010).
Extraction and measurement of ascorbate and glutathione contents
Leaves (0.5 g) were first homogenized in meta-phosphoric acid (5%) containing 1 mM ethylenediaminetetraacetic acid (EDTA). After centrifugation at 11,500×g, the supernatant was used to assay ascorbate and glutathione levels. The level of AsA was estimated following the technique of Nahar et al. (2016a). To determine total ascorbate, the oxidized fraction was reduced by adding 0.1 M dithiothreitol, and absorbance was measured at 265 nm (using 1 unit ascorbate oxidase, AO). The oxidized form of AsA, the dehydroascorbate (DHA) content, was calculated by subtracting reduced AsA from the total amount of AsA. The glutathione pool was assayed according to previously described procedures (Yu et al. 2003; Nahar et al. 2016a) using a standard curve for GSH and GSSG with a known concentration. The GSH content was calculated by subtracting GSSG from total GSH.
Enzymes assays
The protein concentration of each sample was first determined according to Bradford (1976). A 0.5 g of leaves from each treatment were homogenized (in 50 mM K–P, pH 7.0 with 100 mM KCl, 1 mM AsA, 5 mM β-mercaptoethanol, and 10% glycerol). The samples were centrifuged at 11,500×g, and the supernatants were collected. If the supernatant was not clear, the samples were centrifuged again; clear supernatants were used for further procedures. The activities of following enzymes were determined according to the procedure of Hasanuzzaman et al. (2011) and Nahar et al. (2016a) or the studies cited therein: lipoxygenase (LOX; EC 1.13.11.12), APX (EC: 1.11.1.11); MDHAR (EC: 1.6.5.4); DHAR (EC: 1.8.5.1), GR (EC: 1.6.4.2), SOD (EC 1.15.1.1); CAT (EC: 1.11.1.6); GPX (EC: 1.11.1.9); GST (EC: 2.5.1.18); Gly I (EC: 4.4.1.5) and Gly II (EC: 3.1.2.6).
Methylglyoxal contents
Leaves were thoroughly homogenized in 5% perchloric acid followed by centrifugation at 11,000×g. The obtained supernatant was decolorized and neutralized. Sodium dihydrogen phosphate and N-acetyl-l-cysteine were added to the neutralized solution, for a final volume of 1 mL. After 10 min, the product N-α-acetyl-S-(1-hydroxy-2-oxo-prop-1-yl) cysteine was spectrophotometrically assessed at 288 nm (Wild et al. 2012).
Statistical analysis
Data were subjected to analysis of variance (ANOVA). Mean differences were compared following Tukey’s honest significance test (HSD) using the XLSTAT v. 18.07 software (Addinsoft 2016). Differences at P ≤ 0.05 were considered significant.
Results
Chlorophyll contents
Although 15% PEG did not significantly reduce the chl a content, the content decreased by 38% compared to control when grown under the effects of 30% PEG (Table 1). In contrast, the chl b content was reduced by both 15 and 30% PEG, at 22 and 33%, respectively, lower than the control. As a result, the chl (a + b) content also decreased by 17 and 36% at 15 and 30% PEG, respectively, though the chl a/b ratio was unaffected. Importantly, application of the NO donor or Arg resulted in significant enhancement in chl a, chl b and chl (a + b) contents, especially under severe drought (Table 1).
Table 1.
Chlorophyll contents in wheat leaves induced by nitric oxide donor and arginine under drought stress conditions
| Treatment | Chlorophyll content (mg g−1 fresh weight) | chl a/b ratio | |||
|---|---|---|---|---|---|
| Treatment | chl a | chl b | chl (a + b) | ||
| Control | Alone | 0.53 ± 0.04 abc | 0.23 ± 0.01 abc | 0.76 ± 0.04 abc | 2.34 ± 0.20 a |
| +SNP | 0.64 ± 0.05 a | 0.24 ± 0.01 a | 0.87 ± 0.05 a | 2.66 ± 0.23 a | |
| +Arg | 0.57 ± 0.04 ab | 0.23 ± 0.01 ab | 0.80 ± 0.05 ab | 2.49 ± 0.21 a | |
| 15% PEG | Alone | 0.45 ± 0.03 c | 0.18 ± 0.01 de | 0.63 ± 0.04 d | 2.53 ± 0.22 a |
| +SNP | 0.51 ± 0.04 bc | 0.20 ± 0.01 bcd | 0.71 ± 0.04 bcd | 2.57 ± 0.22 a | |
| +Arg | 0.49 ± 0.04 bc | 0.22 ± 0.01 abc | 0.71 ± 0.04 bcd | 2.18 ± 0.19 a | |
| 30% PEG | Alone | 0.33 ± 0.03 d | 0.15 ± 0.01 e | 0.48 ± 0.03 e | 2.16 ± 0.19 a |
| +SNP | 0.45 ± 0.03 c | 0.19 ± 0.01 d | 0.64 ± 0.04 d | 2.40 ± 0.21 a | |
| +Arg | 0.45 ± 0.03 c | 0.20 ± 0.01 cd | 0.65 ± 0.04 d | 2.31 ± 0.20 a | |
Here, SNP and Arg indicate 500 µM sodium nitroprusside and 500 µM l-arginine, respectively. Mean (± SD) was calculated from three replicates for each treatment. In a column, the values with different letters are significantly different at P < 0.05 according to Tukey’s HSD test
Proline contents
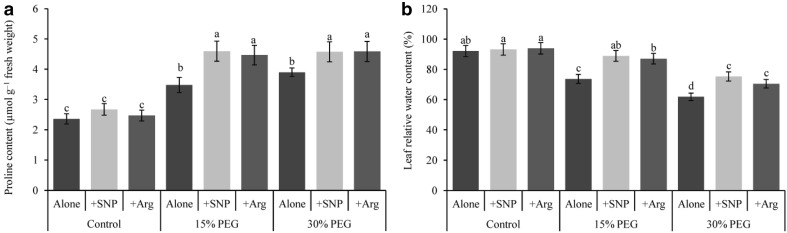
Drought stress at any level markedly elevated the Pro content in leaves, which was 95 and 65% at 15 and 30% PEG, respectively, compared with the control (Fig. 1a). NO or Arg alone did not affect the Pro content significantly; however, the putative effect of exogenous NO and Arg was demonstrated from the further increase in Pro levels in PEG-induced drought-affected seedlings compared with drought stress alone (Fig. 1a).
Fig. 1.
Wheat leaf proline (a) and relative water (b) contents induced by a nitric oxide donor and arginine under drought stress conditions. SNP and Arg indicate 500 µM sodium nitroprusside and 500 µM l-arginine, respectively. The mean (± SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 according to Tukey’s HSD test
Leaf relative water content
Leaf RWC of wheat seedlings decreased by 20 and 33% under mild and severe drought stresses, respectively, compared with control seedlings (Fig. 1b). Nitric oxide and Arg supplementation alleviated these effects of drought stress and restored leaf RWC under both mild and severe drought stresses.
Oxidative stress indicators
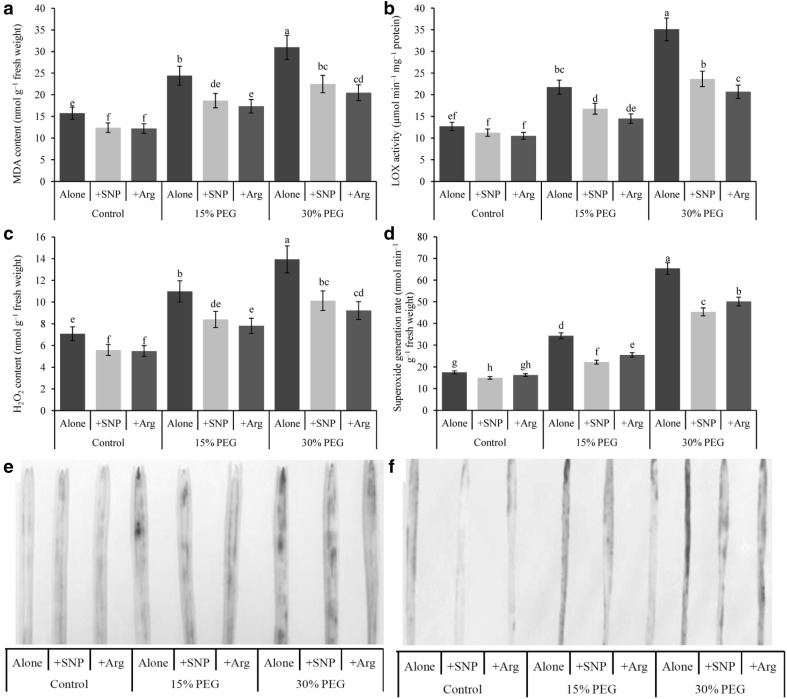
Drought stress at any level increased the membrane lipid peroxidation, as indicated by high MDA contents in drought-stressed wheat seedlings compared with the control (Fig. 2a). Similarly, the activity of LOX increased significantly in drought-exposed wheat seedlings compared with control seedlings (Fig. 2b). Compared with the control, Hydrogen peroxide contents increased by 55 and 97% in response to drought stress induced by 15 and 30% PEG, respectively (Fig. 2c). In addition, an increase in the O·−2 generation rate of 96 and 274% was observed with mild and severe drought stresses, respectively, compared with the control (Fig. 2d). Exogenous NO or Arg application in drought-affected wheat seedlings decreased the activity of LOX, the H2O2 content, the O·−2 generation rate and the MDA content compared with drought-affected seedlings without the protectant applied.
Fig. 2.
Wheat leaf MDA content (a), LOX activity (b), H2O2 content (c), O·−2 generation rate (d), DAB staining (e) of H2O2 and NBT staining (f) of superoxide O·−2 induced by a nitric oxide donor and arginine under drought stress conditions. SNP and Arg indicate 500 µM sodium nitroprusside and 500 µM l-arginine, respectively. The mean (± SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 according to Tukey’s HSD test
Histochemical detection of ROS
DAB and NBT staining revealed drought-induced over production of H2O2 and O·−2 in wheat leaves (indicated as dark brown patches and dark blue spots, respectively). The beneficial effects of SNP and Arg were reflected in the reduction of H2O2 brown patches and O·−2 blue spots in drought-affected wheat leaves (Fig. 2e, f).
Ascorbate and glutathione pools
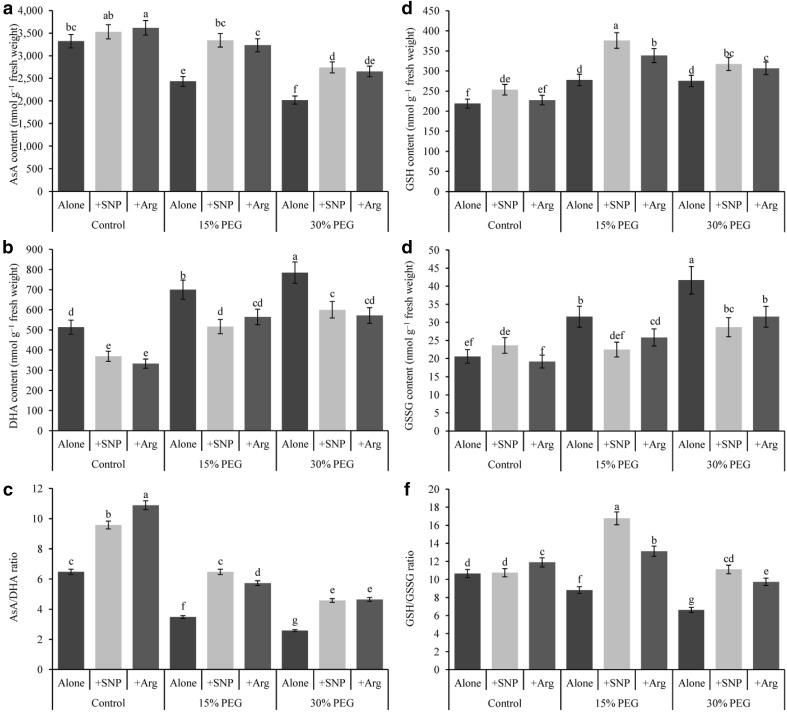
The level of AsA decreased but that of DHA increased notably in response to drought stress induced by 15 and 30% PEG, which subsequently lowered the of AsA/DHA ratio by 46 and 150%, respectively, compared with the control (Fig. 3a–c). Mild and severe drought stresses increased the GSH content by 21 and 27%, increased the GSSG content by 35 and 51% but decreased the GSH/GSSG ratio by 17 and 38%, respectively, compared with the control (Fig. 3d–f). In contrast to drought stress alone, SNP or Arg supplementation increased AsA and GSH contents, decreased DHA and GSSG contents and increased the ratios of AsA/DHA and GSH/GSSG in the drought-affected seedlings.
Fig. 3.
Wheat leaf ascorbate (AsA) content (a), DHA content (b), AsA/DHA ratio (c), GSH content (d), GSSG content (e) and GSH/GSSG ratio (f) induced by nitric oxide donor and arginine under drought stress conditions. SNP and Arg indicate 500 µM sodium nitroprusside and 500 µM l-arginine, respectively. The mean (± SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 according to Tukey’s HSD test
Activities of antioxidant enzymes
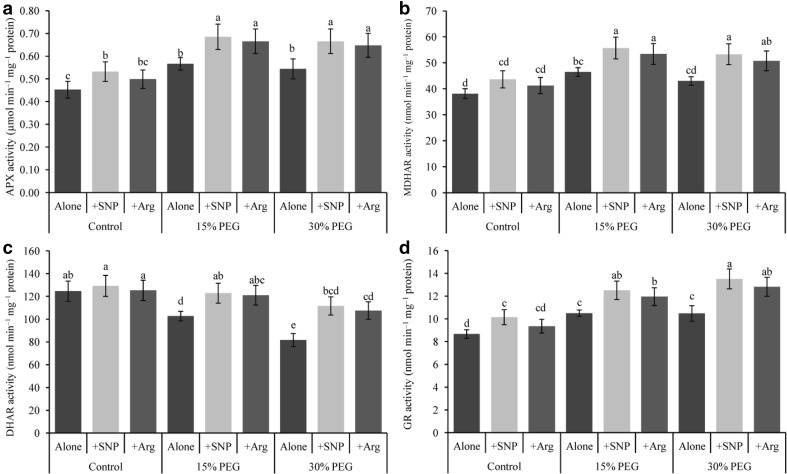
Drought stress enhanced APX, MDHAR and GR activities but decreased that of DHAR compared with the control (Fig. 4a–d). Application of SNP or Arg further enhanced APX, MDHAR, DHAR, and GR activities in drought-affected wheat seedlings compared with drought stress alone.
Fig. 4.
Wheat leaf AsA-GSH cycle enzymes (APX, MDHAR, DHAR and GR) activities induced by a nitric oxide donor and arginine under drought stress conditions. SNP and Arg indicate 500 µM sodium nitroprusside and 500 µM l-arginine, respectively. The mean (± SD) were calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 according to Tukey’s HSD test
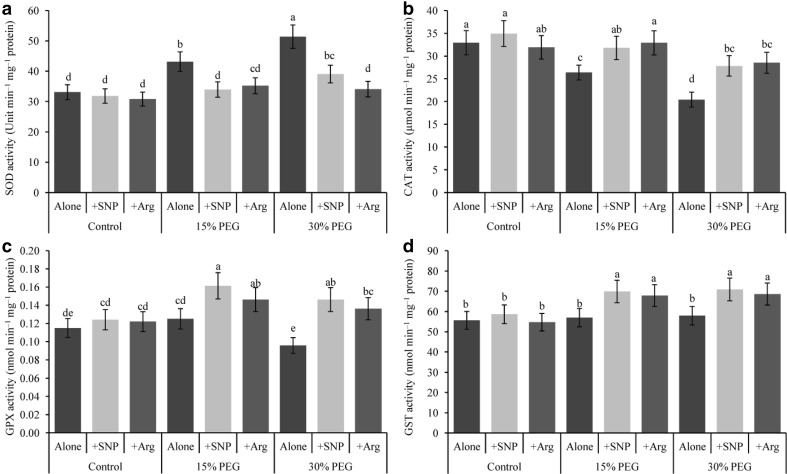
SOD activity increased under drought stress compared with the control (Fig. 5a); however, its activity was reduced when drought-affected seedlings were supplemented with SNP or Arg compared with drought stress alone. Compared with the control, CAT activity decreased at both levels of drought stress (Fig. 5b). Supplementation with SNP and Arg restored and increased CAT activity in drought-stressed wheat seedlings compared with drought stress alone. Drought stress did not change the GPX activity compared with the control (Fig. 5c). In contrast to drought stress treatment alone, SNP or Arg addition increased GPX activity in drought-treated wheat seedlings.
Fig. 5.
Wheat leaf SOD (a), CAT (b), GPX (c) and GST (d) activities induced by a nitric oxide donor and arginine under drought stress conditions. SNP and Arg indicate 500 µM sodium nitroprusside and 500 µM l-arginine, respectively. The mean (± SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 according to Tukey’s HSD test
The activity of GST did not change under drought stress compared with the control (Fig. 5d); however, the addition of SNP or Arg addition did enhance GST activity in drought-treated wheat seedlings.
The glyoxalase system and methylglyoxal levels
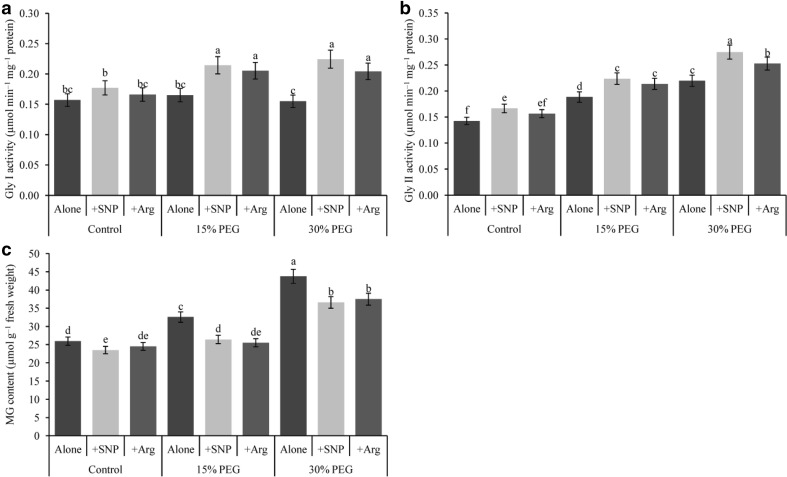
Drought stress increased Gly I activity compared with the control (Fig. 6a) and the addition of SNP or Arg further increased its activity in drought stress treatments. The activity of Gly II increased at both 15 and 30% PEG-induced drought stresses compared with the control (Fig. 6b). Glyoxalase II activity also increased in SNP or Arg-supplemented drought-affected seedlings compared with drought stress alone.
Fig. 6.
Wheat leaf glyoxalase system enzyme (Gly I and Gly II) activities and MG content induced by a nitric oxide donor and arginine under drought stress conditions. SNP and Arg indicate 500 µM sodium nitroprusside and 500 µM l-arginine, respectively. The mean (± SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 according to Tukey’s HSD test
The MG content increased by 26 and 67% under exposure to mild and severe drought stresses, respectively, compared with the control (Fig. 6c). SNP supplementation decreased the MG content by 19 and 16% in 15 and 30% PEG-induced drought stress, whereas Arg supplementation decreased the MG content by 22 and 14% in seedlings under 15 and 30% PEG-induced drought stress, respectively, compared with drought stress alone.
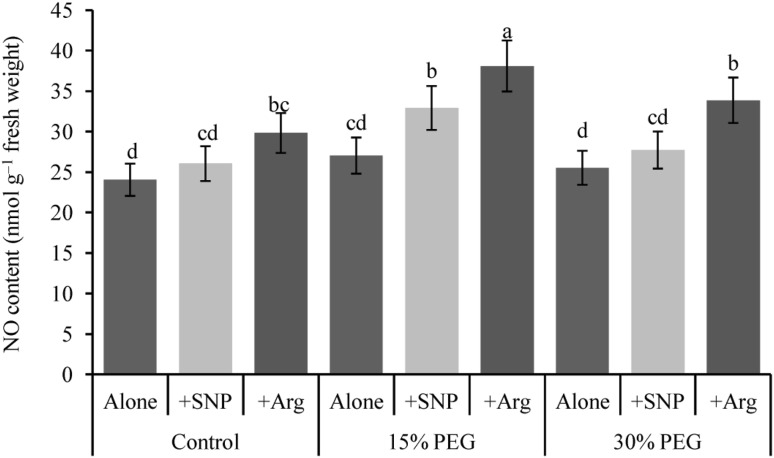
Nitric oxide content
The endogenous NO content increased slightly after SNP or Arg application without drought stress compared with the control (Fig. 7). In addition, the NO content showed a slight increase under mild drought stress compared with the control. SNP or Arg supplementation significantly increased the endogenous NO levels in response to both mild and severe drought stresses compared with drought stress alone.
Fig. 7.
Wheat leaf endogenous nitric oxide content induced by nitric oxide donor and arginine under drought stress conditions. Here, SNP and Arg indicate 500 µM sodium nitroprusside and 500 µM l-arginine, respectively. The mean (± SD) were calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 according to Tukey’s HSD test
Discussion
One of the major consequences of drought stress is chlorophyll reduction. In our study, severe drought stress reduced both chl a and chl b contents, possibly due to impairment of chl biosynthesis. However, exogenous NO and Arg resulted in an augmented chl content. Nitric oxide-induced improvement in chl biosynthesis has been reported recently (Gan et al. 2015; Hatamzadeh et al. 2015). Water loss prevention and maintenance of tissue water content is the most important strategy and a prerequisite for drought tolerance (Gonzalez and Gonzalez-Vilar 2001). Nonetheless, drought tolerance is often associated with osmotic adjustment, and tolerant plants have a better capacity for osmotic adjustment (Zivcak et al. 2009, 2016; Petrov et al. 2017). In this study, exogenous NO or Arg treatment prevented water loss and restored leaf RWC in drought-affected wheat seedlings. Proline acts as an osmoprotectant in maintaining and improving the water status of plants. Free Proline as an antioxidant protects against free radical damage by maintaining a cytosolic atmosphere favorable for the production of biomolecules that function in the stress adaptation process (Nahar et al. 2016b). Supplementation of drought-affected seedlings with SNP or Arg significantly increased the Pro content, which might further influence osmoregulation and water status restoration, increasing the leaf RWC of wheat seedlings. The results of the present study corroborate previous findings for SNP (Arasimowicz-Jelonek et al. 2009) and Arg (Nasibi et al. 2013).
Drought stress inactivates Calvin cycle enzymes, reduces the efficiency of the carboxylation reaction and slows RuBisCO activity and thus, CO2 fixation; all of these processes cause the ROS overproduction. Increased photorespiration is a common effect of drought, which is also responsible for the overproduction of ROS (Hoekstra et al. 2001; Monakhova and Chernyadev 2002). In the present study, histochemical staining of leaves was used for detecting H2O2 and O·−2 . Enhanced ROS production at the cellular level is indicative of oxidative stress in response to drought stress, and increases in ROS and LOX activities reduces lipid peroxidation in membranes. The pattern of ROS production and damage observed in our study were identical to the results of previous studies (Wang et al. 2005; Jiang et al. 2013). SNP or Arg application reduced the ROS content, LOX activity and lipid peroxidation in drought-affected wheat seedlings. NO directly scavenges ROS and can convert O·−2 to a less toxic compound (Laspina et al. 2005). Moreover, NO reacts with lipid alkoxyl (LO) and peroxyl (LOO·) radicals to hinder radical-mediated lipid oxidation (Beligni and Lamattina 1999), and NO-induced modulation of lipid peroxidation by LOX inhibition has been reported (Shi et al. 2007). Previous studies have also shown that NO prevents proteins/antioxidant enzymes from damage (Shi et al. 2007; Alcázar et al. 2010) and enhances their activities (Hasanuzzaman et al. 2011). Exogenous SNP reduces oxidative damage in ginger plants under chill stress (Li et al. 2014), and NO alleviates oxidative effects in cucumber seedlings subjected to drought stress (Arasimowicz-Jelonek et al. 2009). In addition, application of Arg significantly decreases H2O2 contents, LOX activity and oxidative stress in nickel stress-induced Hyoscyamus niger (Nasibi et al. 2013).
Plants possess a group of non-enzymatic and enzymatic antioxidant components for readily scavenging the overproduced ROS. Ascorbate, the most abundant water-soluble non-enzymatic antioxidant, proficiently scavenges O·−2 and OH· (Gill and Tuteja 2010). The AsA content decreases whereas that of DHA increases in wheat seedlings under drought stress. Ascorbate is oxidized to DHA after scavenging ROS, which decreases the AsA/DHA ratio under drought exposure, which is indicative of an inefficient defense. In our study, enhanced APX activity in drought-exposed seedlings was correlated with a reduced AsA content (compared with control treatment). Unaltered MDHAR activity and decreased DHAR activity may be responsible for the reduced AsA content of drought-affected seedlings because both MDHAR and DHAR are AsA recycling enzymes (Gill and Tuteja 2010). Addition of SNP or Arg to drought-stress seedlings enhanced APX, MDHAR and DHAR activities, which restored the AsA level. Similar roles of SNP in enhancing elements of the AsA–GSH cycle have been demonstrated by Hasanuzzaman et al. (2011). SNP promoted cold-tolerance in ginger seedlings by increasing AsA and GSH contents, AsA/DHA and GSH/GSSG ratios and activities of enzymes (APX, DHAR, MDHAR and GR) in the AsA–GSH cycle (Li et al. 2014). Exogenous Arg also augmented APX activity in nickel-exposed H. niger, (Nasibi et al. 2013).
GSH is a non-protein, sulfhydryl-bearing antioxidant with an essential role in stress protection, including drought tolerance (Gill and Tuteja 2010; Nahar et al. 2015). The level of GSSG in this study was markedly increased by drought. The participation of GSH in the ROS scavenging process triggers its conversion to GSSG; thus, GSSG levels increase in the drought exposed seedlings. The activity of GR recycles GSH from GSSG (Gill and Tuteja 2010) and a decrease in GR activity in drought-stressed wheat seedlings is also responsible for reducing the GSH content. Seedlings treated with SNP or Arg showed decreased GSSG levels and increased GR activity and GSH levels under drought stress; after the addition of SNP or Arg, the reduced ratio of GSH/GSSG due to drought (compared to control) increased again compared with drought stress alone. These results are comparable with the findings of previous experiments involving SNP (Li et al. 2014). However, the role of Arg in regulating these components has rarely been demonstrated.
SOD catalyzes conversion of O·−2 to H2O2 (Zhang et al. 2011), and drought-affected wheat plants showed higher SOD activity, corroborating the findings of a previous study examining other stresses (Zhang et al. 2011). However, drought affected seedlings treated with SNP or Arg did not exhibit a further increase in SOD activity. The reduced CAT activity in drought-affected seedlings was due to the higher generation of H2O2 beyond scavenging capacity, which significantly increased the H2O2 content (Nahar et al. 2015). CAT activity was increased in drought-affected wheat seedlings when these plants were treated with SNP or Arg. CAT activity is related to a reduction in H2O2 content. For example, Nasibi et al. (2013) observed exogenous Arg-induced regulation of CAT activity in H. niger under nickel stress. SNP-induced high CAT activity was previously reported in wheat (Hasanuzzaman et al. 2011).
Hydrogen peroxide is generated in various ways and is scavenged by the activity of CAT and GPX as well as elements of the AsA–GSH cycle. Indeed, the AsA–GSH cycle plays a pivotal role in the ROS scavenging process (Neill et al. 2002a, b).
Drought-affected wheat seedlings displayed no changes in GPX activities compared with the control, and this phenomenon was correlated with the generation of high levels of H2O2, similar to earlier reports (Hu et al. 2009; Hasanuzzaman et al. 2012). Compared with drought stress alone, SNP or Arg supplementation increased GPX activities in seedlings affected by drought. GPX activity is vital for reducing H2O2. The exogenous addition of SNP unregulated the activity of GPX in wheat seedlings subjected to salinity (Hasanuzzaman et al. 2011).
GSTs, which are vital enzymes involved in xenobiotic detoxification, catalyze the conjugation of GSH to various toxic compounds (Gill and Tuteja 2010). Drought stress increased GST activity in wheat seedlings, and a similar increase was reported previously (Nahar et al. 2015). A further increase in GST activity due to SNP and Arg addition was observed in drought-affected wheat seedlings, indicating the protective effects of these molecules. Exogenous NO enhances GST activity in salinity-exposed wheat plants (Hasanuzzaman et al. 2011). Exogenous Arg also increased GST activity in drought-stressed wheat seedlings, suggesting that Arg promotes drought stress.
Similar to other abiotic stresses, the increase in MG content reveals its toxicity in drought-affected plants (Nahar et al. 2015). In our study, wheat seedlings showed a marked increase in MG content, which increased with enhanced drought intensity. Drought-affected wheat seedlings treated with SNP or Arg exhibited enhanced Gly I and Gly II activities and high GSH levels, which decreased the MG content. Exogenous SNP was found to modulate components of the glyoxalase system in drought-affected mung bean plants to reduce MG toxicity (Nahar et al. 2016b). The exogenous addition of Arg to drought-stressed wheat seedlings enhanced the glyoxalase system components, which played significant roles in reducing MG content.
Although the endogenous NO content did not increase significantly under drought stress compared with the control, the endogenous level of NO did increase significantly in SNP or Arg-treated drought-affected seedlings. It has been reported that SNP application increases the endogenous NO content in many plant species grown under environmental stresses (Groppa et al. 2008; Li et al. 2014), and endogenous NO production from l-arginine has been reported (Corpas et al. 2006). The higher content of endogenous NO might have played important roles in signaling related to the function of antioxidant systems, ROS detoxification and drought stress adaptation, either resistance or tolerance (Polverari et al. 2003; Sung and Hong 2010).
Conclusion
Based on the results of this study, both NO donor and Arg provided protection against drought-induced oxidative stress. A variety of physiological and biochemical responses, including those to biotic and abiotic stresses, are regulated by NO (Wendehenne et al. 2005). These biomolecules have biological functions either as signaling molecules and/or antioxidants. The signaling action of NO triggers redox- and defense-related gene expressions (Polverari et al. 2003; Sung and Hong 2010). However, the induction and enhancement of antioxidant system components, including both non-enzymatic and enzymatic components, by Arg and NO application, have rarely been studied. In particular, there is a scarcity of knowledge regarding the roles of Arg in such contexts. The effect of Arg on PA contents and growth in beans have been studied under salinity stress (Zeid 2009). Moreover, the effect of l-arginine on growth and chl and mineral contents in apples has been reported (Sotiropoulos et al. 2005). Arg acts as a precursor for both PAs and NO, and the biosynthetic pathways of PAs and NO are interconnected through the Arg-dependent pathway. Previous studies highlight the interactive signaling roles of Arg, PAs and NO in modulating an array of biological functions related to the growth/development and the mechanism of stress adaptation, which illustrate new insight into Arg, PAs, and NO research.
Acknowledgements
The first author is grateful to the Japan Society for the Promotion of Science (JSPS), Japan for financial support. We acknowledge Taufika Islam Anee, Mazhar Ul Alam and Farah Tasmin, Laboratory of Plant Stress Responses, Faculty of Agriculture, Kagawa University, Japan for the critical reading and formatting of this manuscript.
Author contributions
M.H., M.F. and H.O. conceived and designed the experiments; M.H., K.N. and A.R. performed the experiments; M.H. and M.I. analyzed the data; M.F., M.I. and H.O. contributed reagents/materials/analysis tools; K.N. and M.H. wrote the manuscript. All authors have read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Mirza Hasanuzzaman, Email: mhzsauag@yahoo.com.
Masayuki Fujita, Phone: +81878913033, Email: fujita@ag.kagawa-u.ac.jp.
References
- Addinsoft . XLSTAT V. 2016.04.32525: data analysis and statistics software for Microsoft Excel. Paris: Addinsoft; 2016. [Google Scholar]
- Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta. 2010;231:1237–1249. doi: 10.1007/s00425-010-1130-0. [DOI] [PubMed] [Google Scholar]
- Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Kubiś J. Interaction between polyamine and nitric oxide signaling in adaptive responses to drought in cucumber. J Plant Growth Regul. 2009;28:177–186. doi: 10.1007/s00344-009-9086-7. [DOI] [Google Scholar]
- Arnon DT. Copper enzymes in isolated chloroplasts polyphenaloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teari D. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta. 1999;208:337–344. doi: 10.1007/s004250050567. [DOI] [Google Scholar]
- Belkheiri O, Mulas M. Effect of water stress on growth, water use efficiency and gas exchange as related to osmotic adjustment of two halophytes Atriplex spp. Funct Plant Biol. 2013;40:466–474. doi: 10.1071/FP12245. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen F, Wang F, Wu F, Mao W, Zhang G, Zhou M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem. 2010;48:663–672. doi: 10.1016/j.plaphy.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, Sandalio LM, del Río LA. Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta. 2006;224:246–254. doi: 10.1007/s00425-005-0205-9. [DOI] [PubMed] [Google Scholar]
- Freedland RA, Crozier GL, Hicks BL, Meijer AJ. Arginine uptake by isolated rat liver mitochondria. Biochim Biophys Acta. 1984;802:407–412. doi: 10.1016/0304-4165(84)90357-X. [DOI] [PubMed] [Google Scholar]
- Freschi L. Nitric oxide and phytohormone interactions: current status and perspectives. Front Plant Sci. 2013;4:398. doi: 10.3389/fpls.2013.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Wu X, Zhong Y. Exogenously applied nitric oxide enhances the drought tolerance in hulless barley. Plant Prod Sci. 2015;18:52–56. doi: 10.1626/pps.18.52. [DOI] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Gonzalez-Vilar M. Determination of relative water content. In: Roger MJR, editor. Handbook of plant ecophysiology techniques. Amsterdam: Springer; 2001. pp. 207–212. [Google Scholar]
- Groppa MD, Rosales EP, Iannone MF, Benavides MP. Nitric oxide, polyamines and Cd-induced phytotoxicity in wheat roots. Phytochem. 2008;69:2609–2615. doi: 10.1016/j.phytochem.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Hao GP, Zhang JH. The role of nitric oxide as a bioactive signaling molecule in plants under abiotic stress. In: Hayat S, Mori M, Pichtel J, Ahmad A, editors. Nitric oxide in plant physiology. Weinheim: Wiley; 2010. pp. 115–138. [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Physiological and biochemical mechanisms of nitric oxide induced abiotic stress tolerance in plants. Am J Plant Physiol. 2010;5:295–324. doi: 10.3923/ajpp.2010.295.324. [DOI] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 2011;5:353–365. doi: 10.1007/s11816-011-0189-9. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Teixeria da Silva JA, Fujita M. Plant responses and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Bandi V, Shanker AK, Shanker C, Mandapaka M, editors. Crop stress and its management: perspectives and strategies. Berlin: Springer; 2012. pp. 261–316. [Google Scholar]
- Hasanuzzaman M, Nahar K, Gill SS, Fujita M. Drought stress responses in plants, oxidative stress and antioxidant defense. In: Tuteja N, Gill SS, editors. Climate change and plant abiotic stress tolerance. Weinheim: Wiley; 2014. pp. 209–250. [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam MM, Bhuyan MHMB, Oku H, Fujita M. Exogenous nitric oxide pretreatment protects Brassica napus L. seedlings from paraquat toxicity through the modulation of antioxidant defense and glyoxalase systems. Plant Physiol Biochem. 2018;126:173–186. doi: 10.1016/j.plaphy.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Oku H, Nahar K, Bhuyan MHMB, Bhuyan MHMB, Mahmud JA, Baluska F, Fujita M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol Repo. 2018 doi: 10.1007/s11816-018-0480-0. [DOI] [Google Scholar]
- Hatamzadeh A, Molaahmad Nalousi A, Ghasemnezhad M, Biglouei MH. The potential of nitric oxide for reducing oxidative damage induced by drought stress in two turfgrass species, creeping bentgrass and tall fescue. Grass Forage Sci. 2015;70:538–548. doi: 10.1111/gfs.12135. [DOI] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001;6:431–438. doi: 10.1016/S1360-1385(01)02052-0. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ge Y, Zhang C, Ju T, Cheng W. Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Regul. 2009;59:51–61. doi: 10.1007/s10725-009-9387-7. [DOI] [Google Scholar]
- Jiang J, Su M, Chen Y, Gao N, Jiao C, Sun Z, Li F, Wang C. Correlation of drought resistance in grass pea (Lathyrus sativus) with reactive oxygen species scavenging and osmotic adjustment. Biologia. 2013;68:231–240. doi: 10.2478/s11756-013-0003-y. [DOI] [Google Scholar]
- Kirkham MB. Principles of soil and plant water relations. Amsterdam: Elsevier; 2005. [Google Scholar]
- Kocheva KV, Kartseva T, Landjeva S, Georgiev GI. Physiological response of wheat seedlings to mild and severe osmotic stress. Cereal Res Commun. 2009;37:199–208. doi: 10.1556/CRC.37.2009.2.6. [DOI] [Google Scholar]
- Laspina NV, Groppa MD, Tomaro ML, Benavides MP. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 2005;169:323–330. doi: 10.1016/j.plantsci.2005.02.007. [DOI] [Google Scholar]
- Li X, Gong B, Xu K. Interaction of nitric oxide and polyamines involves antioxidants and physiological strategies against chilling-induced oxidative damage in Zingiber officinale Roscoe. Sci Hortic. 2014;170:237–248. doi: 10.1016/j.scienta.2014.03.026. [DOI] [Google Scholar]
- Monakhova OF, Chernyadev II. Protective role of kartolin-4 in wheat plants exposed to soil drought. Appl Environ Microbiol. 2002;38:373–380. [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defense and methylglyoxal detoxification systems. AoB Plants. 2015;7:1–18. doi: 10.1093/aobpla/plv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Mahmud JA, Suzuki T, Fujita M. Polyamines confer salt tolerance in mung bean by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense and methylgyoxal detoxification systems. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M. Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf. 2016;126:245–255. doi: 10.1016/j.ecoenv.2015.12.026. [DOI] [PubMed] [Google Scholar]
- Nasibi F, Heidari T, Asrar Z, Mansoori H. Effect of arginine pre-treatment on nickel accumulation and alleviation of the oxidative stress in Hyoscyamus niger. J Soil Sci Plant Nutr. 2013;13:680–689. [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hurat RD, Hancock JT. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot. 2002;53:1237–1247. doi: 10.1093/jexbot/53.372.1237. [DOI] [PubMed] [Google Scholar]
- Neill S, Desikan R, Hancock J. Hydrogen peroxide signalling. Curr Opin Plant Biol. 2002;5:388–395. doi: 10.1016/S1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Oz MT, Eyidogan F, Yucel M, Öktem HA. Functional role of nitric oxide under abiotic stress conditions. In: Khan MN, Mobin M, Mohammad F, Corpasn FJ, editors. Nitric oxide action in abiotic stress responses in plants. New York: Springer; 2015. pp. 21–41. [Google Scholar]
- Petrov P, Petrova A, Dimitrov I, Tashev T, Olsovska K, Brestic M, Misheva S. Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J Agro Crop Sci. 2017 doi: 10.1111/jac.12255. [DOI] [Google Scholar]
- Polverari A, Molesini B, Pezzotti M, Buonaurio R, Marte M, Delledonne M. Nitric oxide-mediated transcriptional changes in Arabidopsis thaliana. Mol Plant Microbe Interact. 2003;16:1084–1105. doi: 10.1094/MPMI.2003.16.12.1094. [DOI] [PubMed] [Google Scholar]
- Rahman A, Nahar K, Hasanuzzaman M, Fujita M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Hossain MS, Mahmud JA, Nahar K, Hasanuzzaman M, Fujita M. Manganese-induced salt stress tolerance in rice seedlings: regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol Mol Biol Plants. 2016;22:291–306. doi: 10.1007/s12298-016-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheokand S, Kumari A, Sawhney V. Effect of nitric oxide and putrescine on antioxidative responses under NaCl stress in chickpea plants. Physiol Mol Biol Plant. 2008;14:355–362. doi: 10.1007/s12298-008-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Ding F, Wang X, Wei M. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem. 2007;45:542–550. doi: 10.1016/j.plaphy.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Singh HP, Kaur S, Batish DR, Sharma VP, Sharma N, Kohli RK. Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice) Nitric Oxide. 2009;20:289–297. doi: 10.1016/j.niox.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos TE, Dimassi KN, Therios IN. Effects of l-arginine and l-cysteine on growth, and chlorophyll and mineral contents of shoots of the apple rootstock EM 26 cultured in vitro. Biol Plant. 2005;49:443–445. doi: 10.1007/s10535-005-0025-6. [DOI] [Google Scholar]
- Sung CH, Hong JK. Sodium nitroprusside mediates seedling development and attenuation of oxidative stresses in Chinese cabbage. Plant Biotechnol Rep. 2010;4:243–251. doi: 10.1007/s11816-010-0138-z. [DOI] [Google Scholar]
- Tapiero H, Mathé G, Couvreur P, Tew KD. I. Arginine. Biomed Pharmacother. 2002;56:439–445. doi: 10.1016/S0753-3322(02)00284-6. [DOI] [PubMed] [Google Scholar]
- Todd CD, Cooke JEK, Mullen RT, Gifford DJ. Regulation of loblolly pine (Pinus taeda L.) arginase in developing seedling tissue during germination and post-germinative growth. Plant Mol Biol. 2001;45:555–565. doi: 10.1023/A:1010645616920. [DOI] [PubMed] [Google Scholar]
- Wang YS, Yang ZM. Nitric oxide reduces aluminum toxicity by preventing oxidative stressing the roots of Cassia tora L. Plant Cell Physiol. 2005;46:1915–1923. doi: 10.1093/pcp/pci202. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang Q, Kwon S, Kwak S, Su W. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol. 2005;162:465–472. doi: 10.1016/j.jplph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Gould K, Lamotte O, Durner J, Vandelle E, Lecourieux D, Courtois C, Barnavon L, Bentéjac M, Pugin A. NO signaling functions in the biotic and abiotic stress responses. BMC Plant Biol. 2005;5:S3. doi: 10.1186/1471-2229-5-S1-S35. [DOI] [Google Scholar]
- Wild R, Ooi L, Srikanth V, Münch G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-l-cysteine assay. Anal Bioanal Chem. 2012;403:2577–2581. doi: 10.1007/s00216-012-6086-4. [DOI] [PubMed] [Google Scholar]
- Winter G, Todd CD, Trovato M, Forlani G, Funck D. Physiological implications of arginine metabolism in plants. Front Plant Sci. 2015;6:534. doi: 10.3389/fpls.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Sun X, Jin J, Zhou H. Protective effect of nitric oxide on light-induced oxidative damage in leaves of tall fescue. J Plant Physiol. 2010;167:512–518. doi: 10.1016/j.jplph.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK. Methylglyoxal detoxification by glyoxalase system: a survival strategy during environmental stresses. Physiol Mol Biol Plants. 2005;11:1–11. [Google Scholar]
- Yadav SK, Singla-Pareek SL, Sopory SK. An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metabol Drug Interact. 2008;23:51–68. doi: 10.1515/DMDI.2008.23.1-2.51. [DOI] [PubMed] [Google Scholar]
- Yu YG, Weiss RL. Arginine transport in mitochondria of Neurospora crassa. J Biol Chem. 1992;267:15491–15495. [PubMed] [Google Scholar]
- Yu CW, Murphy TM, Lin CH. Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol. 2003;30:955–963. doi: 10.1071/FP03091. [DOI] [PubMed] [Google Scholar]
- Zeid IM. Effect of arginine and urea on polyamines content and growth of bean under salinity stress. Acta Physiol Plant. 2009;31:65–70. doi: 10.1007/s11738-008-0201-3. [DOI] [Google Scholar]
- Zhang XL, Jia XF, Yu B, Gao Y, Bai JG. Exogenous hydrogen peroxide influences antioxidant enzyme activity and lipid peroxidation in cucumber leaves at low light. Sci Hortic. 2011;129:656–662. doi: 10.1016/j.scienta.2011.05.009. [DOI] [Google Scholar]
- Zheng C, Jiang D, Liu F, Dai T, Liu W, Jing Q, Cao W. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ Exp Bot. 2009;67:222–227. doi: 10.1016/j.envexpbot.2009.05.002. [DOI] [Google Scholar]
- Zivcak M, Repková J, Olšovská K, Brestič M. Osmotic adjustment in winter wheat varieties and its importance as a mechanism of drought tolerance. Cereal Res Commun. 2009;37:569–572. [Google Scholar]
- Zivcak M, Brestic M, Sytar O. Osmotic adjustment and plant adaptation to drought stress. In: Hossain MA, Wani S, Bhattacharjee S, Burritt D, Tran LS, editors. Drought stress tolerance in plants. Cham: Springer; 2016. pp. 105–143. [Google Scholar]