Abstract
Discrepancies regarding the link between autonomic nervous system (ANS) activity and psychopathology may be due in part to inconsistent measurement of non-psychological factors, including eating, drinking, activity, posture, and interacting with others. Rather than sources of noise, behaviors like being active and being with others may be the behavioral pathways that connect psychopathology symptoms to autonomic activity. The present study examined whether behaviors mediate the association of depression, anxiety, and hypomanic traits with ANS by using experience sampling methodology and ambulatory impedance cardiography. Participants (n = 49) completed measures of affect and one day of experience sampling and ambulatory impedance cardiography. The association of hypomanic traits with heart rate variability and heart rate was mediated by physical activity, and social activity mediated the association of depressive symptoms and respiration. These results highlight the importance of considering the pathways between psychopathology and ANS and the mediating role that everyday behaviors play.
Keywords: heart rate variability, impedance cardiography, RMSSD, PEP, ambulatory assessment, experience sampling methodology, ambulatory psychophysiology
1. Introduction
The autonomic nervous system (ANS) is widely linked to health and psychological functioning. Specifically, under conditions of stress, dysregulation of the ANS can potentially increase vulnerability for psychopathology (Beauchaine, 2001). Psychophysiology has been used to examine the contributions of the two branches of the ANS: the sympathetic system, responsible for mobilization, and the parasympathetic system, responsible for restoration. The cardiac pre-ejection period (PEP), a measure of contractility that reflects beta-adrenergic sympathetic impact on the heart, is a common measure of sympathetic activity (Kelsey, 2012), especially in research on effort and motivational engagement (Obrist, 1976; Silvia et al., 2013). Heart rate variability (HRV) has been used as an index of parasympathetic activity or vagal control, and it has been proposed to be a physiological index of emotional functioning (Gruber et al., 2011; Kogan et al., 2013; Oveis et al., 2009).
Changes to the dynamic balance of the sympathetic and parasympathetic systems have been associated with both personality and psychopathology. Specifically, studies have examined the associations of HRV with depression, anxiety, and hypomania/mania, and found that affective disorders are linked to increased risk for cardiovascular disorders (Gorman and Sloan, 2000). Anxiety is robustly associated with dysregulation of the ANS, particularly with reduced HRV (Thayer et al., 1996). A recent meta-analysis reported that anxiety disorders (specifically panic disorder, post-traumatic stress disorder, generalized anxiety disorder, and social anxiety disorder) and trait measures of anxiety are associated with reduced high-frequency HRV with small to moderate effects (Miu et al., 2009).
Findings are more mixed regarding depression and ANS, with several studies finding decreased HRV and others finding no differences between depressed people and healthy controls. Recent meta-analyses of HRV in depression, however, reported a small to medium effect of depression on HRV, suggesting that depression, like anxiety, was associated with decreased vagal control of heart rate and respiration (decreased HRV) during increased environmental demands (Kemp et al., 2010; Rottenberg, 2007). Note that Rottenberg (2007) highlighted the difficulty of parsing the effects of reduced HRV on depression given its comorbidity with anxiety.
For bipolar spectrum psychopathology and hypomania/mania, the literature is mixed. Studies of bipolar disorder have variously reported elevated HRV (Gruber et al., 2015, 2011; Kang and Gruber, 2013), decreased HRV and increased LF/HF ratio (proposed to reflect the sympathovagal balance; Chang et al., 2014; Henry et al., 2010; Levy, 2014; Quintana et al., 2016), decreased HRV and decreased LF/HF ratio (Cohen et al., 2003), decreased HRV and no differences in the LF/HF ratio (Moon et al., 2013), and no differences in HRV (Todder et al., 2005). In addition, several studies have found that decreased HRV was associated with severity of mania symptoms (Chang et al., 2014; Henry et al., 2010). Thus, it is unclear whether bipolar spectrum psychopathology is associated with increased or decreased HRV or whether this varies as a function of context.
Significantly less work has examined the association of the sympathetic nervous system with personality or psychopathology. Previous studies have suggested that increased risk for cardiovascular and metabolic disease in anxiety and depression may be associated with increased activity of the sympathetic nervous system (Glassman, 2008; Penninx et al., 2001), whereas others suggest no changes or decreased activity of the sympathetic nervous system associated with psychopathology. The majority of studies that have found decreased activity of the sympathetic system have concentrated on the link between effort and depression (Gendolla et al., 2012; Silvia et al., 2013). Silvia et al. (2016) specifically found that depression was associated with reduced effort, as indexed by lower reactivity of the sympathetic nervous system (pre-ejection period; PEP), during difficult tasks. In contrast, others have reported that PEP was unrelated to trait anxiety during a stressful task (Burns et al., 1992). Of note, inconclusive results regarding sympathetic nervous system functioning and depression and anxiety may be in part due to medication effects (Licht et al., 2012).
Increasing evidence suggests that the discrepancies in the literature regarding the link between the ANS and personality or psychopathology may be in part due to inconsistent measurement of non-psychological factors (Houtveen and de Geus, 2009). Everyday behaviors that exist in naturalistic settings highly influence cardiac functioning but are outside of experimental control. Yet, most laboratory-based psychophysiological studies typically hold them constant at some level (e.g., requiring all participants to be seated). However, Houtveen and de Geus argue that researchers need to examine physiological processes in more naturalistic settings to better understand the role that day-to-day behaviors play in the measurement of the parasympathetic and sympathetic influences and their links to outcomes of interest.
Behaviors known to affect measures of sympathetic and parasympathetic systems include physical activity and posture (Houtveen et al., 2005), diurnal variation and sleep (van Eekelen et al., 2004), speech (Reilly and Moore, 2003; Weber and Smith, 1990), and nicotine use (Houtveen and de Geus, 2009). Factors like these are not merely “noise” but rather behavioral and lifestyle factors that may, in part, carry the effects of psychopathology symptoms on physiological activity. Stated differently, factors like depression and anxiety might predict daily physiology by virtue of their effects on people’s everyday activities like how much they move, whether are alone or with others, and how often they are eating, drinking, and smoking.
Previous research examining links between the ANS and psychopathology has largely emphasized the common neurobiological mechanisms shared between psychopathology and ANS functioning (Thayer et al., 1998). Given the lack of evidence that ANS dysregulation is a biomarker for the development of psychopathology, it is perhaps more important to understand the relationship between clinical symptoms of a disorder and ANS functioning. One potential aforementioned behavior that may provide relevant information regarding the connection between psychopathology and ANS functioning is psychomotor activity. Specifically, a common symptom of depression is psychomotor retardation, which may be related to ANS functioning or potentially caused by low ANS functioning (Parker, 2000). It could be that low psychomotor activity seen in depression and ANS dysfunction (low heart rate variability or parasympathetic functioning) are both related to the cholinergic system changes seen in depression (Volkers et al., 2003). Likewise, bipolar disorder is characterized by psychomotor agitation and increases in goal-directed activities and behaviors. Increased physical activity and movement has been associated with lower resting heart rate and higher heart rate variability, indicating more adaptive sympathetic and parasympathetic nervous system functioning (Levy et al., 1998; Rennie et al., 2003). Limbic-frontal projections are crucial for the regulation of goal-directed activities and behavior and are largely related to the availability and firing of dopaminergic and noradrenergic activity (see Grace et al., 2007 for review). Importantly, a recent meta-analysis showed decreased frontal activity and abnormal limbic activity in bipolar disorder (Chen et al., 2011) Perhaps the common mechanism that could explain ANS dysregulation and bipolar disorder exists in underlying dysfunction of limbic-frontal dopaminergic and noradrenergic projections that contribute to goal-directed behaviors and increased psychomotor activity as well as ANS functioning. This thinking would suggest that it is not likely that ANS dysfunction is an underlying predictor of a psychological disorder, but rather a consequence of either central nervous system dysfunction or clinical behaviors related to the central nervous system dysfunction in the disorder of interest. This is not a new hypothesis given earlier theoretical work on the neurovisceral integration model, which proposed that the connection between the central nervous system and autonomic nervous system may set constraints on behaviors given one’s environmental context (Thayer and Lane, 2009, 2000).
One way to examine the interaction between psychological, physiological, and non-psychological factors is through ambulatory assessment, a method by which participants are measured in their normal daily environments (Houtveen and de Geus, 2009). Technological advances now allow for the non-invasive measurement of cardiac function in daily life through ambulatory psychophysiology devices. Specifically, ambulatory devices are able to measure sympathetic and parasympathetic activity as well as heart rate (de Geus and Van Doornen, 1996) based on the extraction of the interbeat-interval time series from the raw electrocardiogram (ECG), respiration, and impedance cardiogram (ICG) signals (Houtveen & de Geus, 2009). Additionally, experience sampling methodology (ESM), a structured daily diary method, can be used to assess participants’ affect, cognition, stress, and behavior in the moment (Csikszentmihalyi and Larson, 2014; Mehl & Conner, 2012). Both of these methods provide high ecological validity to findings regarding personality and psychopathology and allow the research to examine contextual factors—such as eating, being physically active, and spending time with other people—rather than hold them constant.
Studying the behavioral pathways from psychopathology to physiology using ambulatory assessment may help disentangle the relationship of depression, anxiety, and hypomania with autonomic nervous system functioning. The present study was a preliminary examination of these associations in a non-clinically ascertained sample, given that subclinical manifestations of depression, anxiety, and hypomania predict impairment and risk for developing clinical disorders and enable the examination of psychophysiology without the confounds of disease burden (e.g., medication effects). Thus, the present preliminary study had two goals. First, it aimed to examine contextual factors and cardiac function in the real-world outside of the laboratory environment using ESM and ambulatory psychophysiology to improve the ecological validity of previous psychophysiological findings. Second, it aimed to examine the behavioral pathways that might mediate the association between psychopathology and physiology in real world settings. Specifically, it was hypothesized that behaviors in daily life as measured by ESM may account for or mediate the associations between depression, anxiety, and hypomanic personality traits and parasympathetic and sympathetic ANS functioning. Specific directional relationships were not hypothesized because this preliminary study was exploratory in nature.
2. Materials and Methods
2.1. Participants
The study enrolled 49 participants from psychology courses at University of North Carolina at Greensboro (UNCG) for credit toward a voluntary research option. The mean age was 19.3 years (SD = 1.7, range = 18–25 years) and the sample was 77.6% female. Participants were 36.7% Caucasian/European American, 38. 8% African American, 6.1% Hispanic/Latino, 8.2% Asian/Pacific Islander, 4.1% biracial, and 6.1% declined to state their ethnicity. The average Body Mass Index (BMI) (based on self-reported height and weight) was 24.2 (SD = 5.6).
2.2. Materials and procedures
This research was approved by the UNCG Institutional Review Board, and all participants provided informed consent. All participants worked with same-gender researchers. Participants came into the lab and were briefed regarding the purpose of the experiment—to examine how the body responded to normal activities in daily life. Participants were told that after completing measures of personality and individual differences in the lab, they would be connected to an ambulatory physiological recording device during the day and would complete brief self-report surveys throughout on an electronic tablet provided by the lab. The experimenter placed electrodes on the participants and allowed the signals to stabilize. Afterwards, the participants completed demographics and questionnaires. Participants were instructed on how to use the tablets and then sent into their normal daily lives for a period of at least 6 hours.
2.2.1. Psychopathology symptom scales
Depressive symptoms were measured via the 7-item Depression subscale of the Depression Anxiety Stress Scales-21 (DASS-21; Lovibond and Lovibond, 1995). Anxiety symptoms were measured via the 7-item Anxiety subscale of the DASS-21. The DASS-21 uses a 4-point response format from 0 = Did not apply to me at all to 3 = Applied to me very much. The DASS-21 has been used to assess depression, anxiety, and stress in large non-clinical samples (Henry and Crawford, 2005) and has shown validity in distinguishing depression in both clinical and healthy community samples (Antony et al., 1998). Bipolar spectrum psychopathology was measured using the Hypomanic Personality Scale (HPS; Eckblad and Chapman, 1986). The HPS consists of 48 true-false items and taps affective lability, grandiosity, and hypomanic-like behaviors. The HPS was created to measure hypomanic traits in non-clinical samples and has been shown to predict the later development of bipolar spectrum disorders (Eckblad and Chapman, 1986; Kwapil et al., 2000; Walsh et al., 2015).
2.2.2. Experience sampling methodology
Participants completed ESM surveys on 7” Android tablets via the Qualtrics Offline application. The tablets signaled participants at 12 randomized times roughly every 45 minutes (loosely constrained to around 8–9 hours) via a flashing screen and an alarm sound. When signaled, participants opened the Qualtrics Offline survey application and responded to the questions.
To examine the effect of behavioral factors on cardiac functioning, we asked about a range of common behaviors that could affect ambulatory cardiac activity and that might be associated with psychopathology symptoms. Table 1 shows the items and their response scales. For some of the items, people reported on what they were doing during the past 10 minutes, such as their posture (e.g., laying down versus sitting or standing), their social activity (whether they were alone or interacting with other people), and how much they were talking. For other items, people responded based on their behavior since the last signal (roughly 30–40 minutes), such as whether they were eating or drinking, using caffeine or nicotine, or being physically active.
Table 1.
Experience Sampling Items About Behavioral Factors.
| Variable | Item | Response Scale |
|---|---|---|
| Posture | During the past 10 minutes, were you mostly: laying down; sitting down; standing up; walking | 0 (laying down), 1 (sitting down), 2 (standing up), 3 (walking) |
| Talking | In the past 10 minutes, were you talking? | 1 = Not at all, 7 = Very much |
| Social Activity | During the past 10 minutes, were you mostly: alone, by yourself; with other people, but not interacting with them; interacting with other people | 0 (alone, by yourself), 1 (with other people, but not interacting with them), 2 (interacting with other people) |
| Eating | Since the last signal, have you had something to eat? | 0 (No), 1 (Yes) |
| Drinking | Since the last signal, have you had something to drink? | 0 (No), 1 (Yes) |
| Physical Activity | Since the last signal, have you been physically active? | 0 (No), 1 (Yes) |
| Caffeine | Since the last signal, have you had caffeine? | 0 (No), 1 (Yes) |
| Nicotine | Since the last signal, have you had nicotine or smoked? | 0 (No), 1 (Yes) |
After data collection, some of the behavioral factors were omitted from analysis. For example, caffeine use had a high positive correlation with drinking (i.e., people consumed caffeine almost exclusively through caffeinated beverages), so drinking was included as the variable of interest in all analyses. In addition, talking and social activity were highly correlated, so talking was dropped from all analyses. Finally, reported nicotine use was surprisingly low and near zero in our sample, so it too was omitted from subsequent analyses. Thus, the ESM items used as behavioral factors for all analyses were posture, eating, drinking, physical activity, and social activity.
2.2.3. Ambulatory physiological recording
Cardiac autonomic activity was assessed using MindWare Mobile Impedance Cardiograph devices (Model 50–2303–00; Mindware Technologies, Gahanna, OH). Electrocardiogram (ECG) signals were acquired using 3 electrodes placed in a modified Lead II configuration on the right collarbone and both lower ribs. Impedance cardiogram (ICG) signals were acquired using 4 electrodes in a standard tetrapolar configuration. Receiving electrodes were placed on the chest (an upper electrode on the left collarbone and the lower electrode at the base of the sternum). Sending electrodes were placed on the back and displaced 4 cm above and below the receiving electrodes. The devices were clipped to the participant’s clothing (e.g., belt or pants). The signals were acquired at 500 Hz, filtered and amplified internally, and saved locally to an SD card for later analysis.
Ambulatory physiology was assessed continuously. For data analysis, 10 minutes of continuous recording prior to the ESM questionnaire signal were parsed so the ECG and ICG data mapped on to the time period just prior to the self-report survey. Each 10-minute segment was divided into 60-second periods, and the signals were screened and corrected as needed prior to analysis using the Mindware IMP and HRV software. The physiological outcomes were calculated for each 60-second period and then averaged across the 10 minutes for a summary average value for that period. This was done for all the participant’s available physiological segments, up to the maximum of 12 segments during the day.
To assess sympathetic influence on the heart, we measured the cardiac PEP (Kelsey, 2012) and the RZ interval. PEP is the time (in ms) between the ECG Q point (Berntson et al., 2004) and the dZ/dt B point (Lozano et al., 2007). The RZ interval is the time (in ms) between the ECG R point and the dZ/dt Z point (Meijer et al., 2008; van Eijnatten et al., 2014). The points were based on ensemble averages (Kelsey et al., 1998). Q was identified as the lowest point in the 35 ms window preceding R (Berntson et al., 2004); B was estimated via the Lozano et al. (2007) slope/intercept formula (RB = RZ*.55 + 4 ms). RZ is an increasingly used measure of contractility that has the virtues of using the salient R and Z points, so it might be especially suitable for ambulatory recordings (van Lien et al., 2013). RZ has been comparable to PEP in several recent studies (Harper et al., in press; Silvia et al., 2014a, 2014b; Silvia et al., in press).
The primary outcome for parasympathetic influence on the heart was the root mean square of successive interbeat interval differences (RMSSD), a time-domain measure (Schaffer & Ginsberg, 2017) that relies on the measurement of interbeat intervals (IBI). We selected RMSSD because its computation requires only the ECG R-peaks in the IBI series, so it should be relatively robust to potential signal quality issues that could arise in ambulatory assessment. Finally, we calculated heart rate and respiration rate (Ernst et al., 1999).
3. Results
3.1. Analytic approach
The data were analyzed using cluster-robust standard error (CR-SE) models (McNeish et al., 2017; Skrondal and Rabe-Hesketh, 2004). Like hierarchical linear models, CR-SE models correct for the clustering introduced by the repeated measures. These models offer several advantages over multilevel models for the present type of study. First, they afford properly-defined standardized effect sizes (McNeish et al., 2017), even for mediational path models with categorical mediators. Second, many participants had no variance for some of the mediator variables (e.g., their activity level or posture was the same for all assessments), so these variables were “within-person” for some participants but not others. This occasional within-cluster invariance is more easily accommodated with CR-SE models than with multilevel models, which seek to partition variance into two levels. All the behavioral factors (except posture) were treated as categorical when they were outcomes or mediators. The analyses were run in Mplus 8 using maximum likelihood with robust standard errors or with weighted least squares, depending on whether the model had categorical mediators.
Participants completed an average of 8.65 (SD = 2.11, range = 4–12) ESM questionnaires. Descriptive statistics for depression, anxiety, and hypomanic personality as well as the five psychophysiological outcomes are presented in Table 2. Means for the HPS were consistent with prior non-clinical samples collected at UNCG (Kwapil et al., 2011; Sperry and Kwapil, 2017). Means for the DASS were consistent with those reported in non-clinical validation samples (Henry and Crawford, 2005). Hypomanic traits were moderately correlated with depression (r = .39, p = .005) and anxiety (r = .39, p = .005). Not surprisingly, depression and anxiety were highly correlated (r = .76, p < .001).
Table 2.
Descriptive Statistics for Psychopathology Symptoms and Psychophysiological Indicators.
| Mean | SD | Range | |
|---|---|---|---|
| Traits | |||
| Depression | 0.41 | 0.49 | 0–2.29 |
| Anxiety | 0.52 | 0.51 | 0–2.43 |
| Hypomanic Personality | 0.40 | 0.18 | 0.15–0.88 |
| Physiological Indicators | |||
| Respiration Rate | 18.51 | 2.36 | 11.04 – 25.94 |
| Heart Rate | 92.13 | 16.57 | 54.69 – 145.86 |
| RMSSD | 34.54 | 27.02 | 2.91 – 192.61 |
| PEP | 95.78 | 10.64 | 59.75 – 128.20 |
| RZ | 113.36 | 17.31 | 59.20 – 169.80 |
Note. Hypomania scores are the percent of questions endorsed; Depression and Anxiety scores are the mean on a scale from 0 – 3. Means for physiological indicators are averaged across the 12 daily segments. Respiration rate is in cycles per minute; heart rate is in beats per minute; PEP and RZ are in ms.
3.2. Influence of behavioral factors on psychophysiological outcomes
We first examined the relationships between the behavioral factors— posture, eating, drinking, physical activity, and social activity—and the physiological outcomes. Five models were estimated, one for each physiological outcome, and the behavioral factors were entered as simultaneous predictors. Table 3 summarizes the results.
Table 3.
Behavioral Factors Predicting Psychophysiological Outcomes.
| RZ | PEP | RMSSD | HR | Respiration Rate | |
|---|---|---|---|---|---|
| Posture | −.32 (.04)*** | −.27 (.04)*** | −.28 (.05)*** | .43 (.05)*** | .02 (.06) |
| Eating | −.09 (.05) | −.03 (.06) | −.10 (.05)** | .05 (.05) | −.01 (.05) |
| Drinking | .05 (.06) | .03 (.06) | .01 (.05) | .04 (.06) | .02 (.06) |
| Physical Activity | −.02 (.05) | −.05 (.05) | −.03 (.05) | .11 (.06)* | .00 (.05) |
| Social Activity | .11 (.06)* | .13 (.06)** | −.12 (.07)* | .10 (.07) | −.18 (.06)*** |
Note. The regression coefficients are standardized. Standard errors are in parentheses.
p < .10
p < .05
p < .01
Posture predicted several of the ambulatory physiological outcomes. As people’s postures shifted to be more upright, they had higher HR (β = .43, p < .001), shorter PEP intervals (β = −.27, p < .001), shorter RZ intervals (β = −.32, p < .001), and lower RMSSD (β = −.28, p < .001). The pattern collectively suggests higher sympathetic and lower parasympathetic activity during the day as posture changed.
Likewise, social activity during the day predicted several ambulatory physiological outcomes. As people went from being alone to interacting with others, they had slower respiration rates (β = −.18, p = .005), longer PEP intervals (β = .13, p = .038), marginally longer RZ intervals (β = .11, p = .081), and marginally lower RMSSD (β = −.12, p = .065). Taken together, the findings suggest lower sympathetic cardiac activity as social activity increased.
For the remaining behavioral factors, greater physical activity predicted marginally higher HR (β = .11, p = .075) and eating predicted lower RMSSD (β = −.10, p = .039). Drinking had no relationships with physiological outcomes.
3.3. Influence of psychopathology symptoms on behavioral factors
We next examined the relationships between psychopathology symptoms—depression, anxiety, and hypomania symptom scores—and the frequency of the behavioral factors (posture, eating, drinking, physical activity, social activity) in daily life. We ran 5 models, one for each behavioral factor, with the three psychopathology symptoms as predictors. Table 4 summarizes the results.
Table 4.
Psychopathology Symptoms Predicting Daily Behavioral Factors.
| Hypomania | Depression | Anxiety | |
|---|---|---|---|
| Posture | .07 (.05) | −.13 (.07)* | .08 (.07) |
| Eating | −.01 (.05) | −.15 (.09)* | .15 (.09)* |
| Drinking | .01 (.06) | .02 (.06) | −.11 (.06)* |
| Physical Activity | .23 (.11)** | −.08 (.18) | −.19 (.16) |
| Social Activity | .09 (.06) | −.17 (.11) | −.03 (.09) |
Note. The regression coefficients are standardized; standard errors are in parentheses.
p < .10
p < .05
p < .01
Several findings emerged that are consistent with the underlying symptoms. People higher in hypomania were more likely to be physically active during day (β = .23, p = .045), consistent with their higher energy and activity levels. People higher in depression symptoms were more sedentary, in that they were more likely to be laying down or sitting than standing or walking (β = −.13, p = .060); they were marginally less likely to be eating (β = −.15, p = .086). People higher in anxiety symptoms were marginally more likely to be eating (β = .15, p = .082) and less likely to be drinking (β = −.12, p = .064) during the day.
3.4. Influence of psychopathology symptoms on physiological outcomes
Finally, we explored whether depression, anxiety, and hypomanic symptoms directly predicted ambulatory physiological activity during the day. We estimated 5 models, one for each physiological outcome (RZ, PEP, RMSSD, HR, and respiration rate), with depression, anxiety, and hypomanic personality scores as predictors. No marginal or significant relationships were found; Table 5 displays the findings.
Table 5.
Psychopathology Symptoms Predicting Daily Psychophysiological Outcomes.
| Hypomania | Depression | Anxiety | |
|---|---|---|---|
| RZ | −.17 (.13) | −.14 (.12) | .18 (.16) |
| PEP | −.13 (.14) | −.19 (.17) | .19 (.15) |
| RMSSD | .06 (.11) | .01 (.15) | .17 (.14) |
| HR | .00 (.07) | −.02 (.10) | −.15 (.11) |
| Respiration Rate | −.01 (.08) | −.10 (.10) | .11 (.10) |
Note. The regression coefficients are standardized; standard errors are in parentheses.
p < .10
p < .05
p < .01
3.5. Exploring mediational relationships
Thus far, the results have shown that many common behavioral factors, from posture to social activity, affect ambulatory physiological activity in daily life, and that psychopathology symptoms, in turn, predict the likelihood of engaging in several of these behaviors. A natural next step is to explore mediation models that estimate whether behavioral factors act as mediators between psychopathology symptoms and daily physiological activity. Given the large set of predictors, mediators, and outcomes, we narrowed the set of variables based on the prior analyses. First, any behaviors that were significantly related to either psychophysiological functioning or depression, anxiety, and hypomanic personality were included in the mediation models. Second, any behavioral factors that had a standardized effect size greater than 0.15 were included. Five models were run for each personality trait (1 for each outcome, for 15 models total). The CR-SE models do not afford the higher-powered significance tests of indirect effects, such as bias-corrected bootstrapping (Fritz and MacKinnon, 2007), but the available Sobel test was conducted to provide some information.
3.5.1. Hypomania
For hypomanic personality, posture and physical activity were chosen to mediate the five psychophysiological outcomes. Physical activity mediated hypomanic personality and RMSSD (see Figure 1). As hypomania increased, people were significantly more likely to report being physically active during the day (β = .22, p = .028). Higher physical activity, in turn, predicted significantly lower RMSSD (β = −.19, p = .010; indirect effect β = −.04 [−.10, .01], p = .121).
Figure 1.
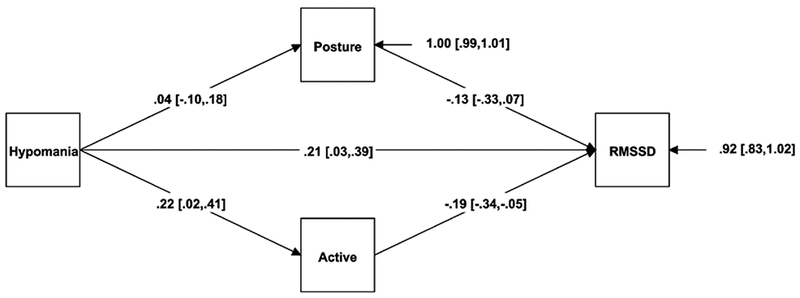
Mediation model for hypomanic personality, physical activity, and RMSSD.
Note. The regression coefficients are standardized; 95% confidence intervals are in brackets.
A similar pattern appeared for heart rate (see Figure 2). As hypomania increased, people were more likely to report being physically active during the day (β = .22, p = .028), and higher physical activity, in turn, significantly predicted higher HR (β = .20, p = .017; indirect effect β = .04 [−.01, .10], p = .092).
Figure 2.
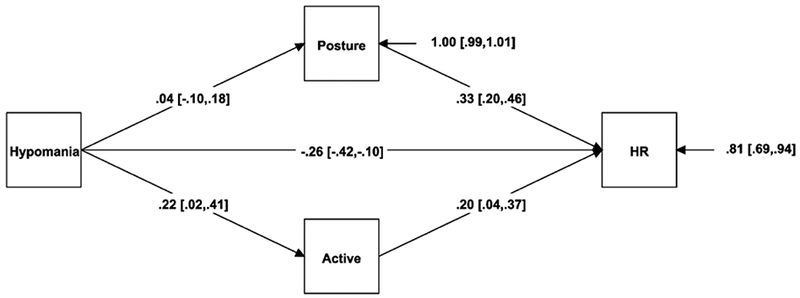
Mediation model for hypomanic personality, physical activity, and HR.
Note. The regression coefficients are standardized; 95% confidence intervals are in brackets.
3.5.2. Depression
For depression, posture, eating, and social activity were included to predict the five psychophysiological outcomes. Figure 3 illustrates the mediating role of social activity as a mediator between depression and respiration rate. As depression scores increased, people were more likely to be alone and less likely to be interacting with others (β = −.18, p = .048). In turn, as people interacted with others, their respiration rate declined (β = −.23, p = .001; indirect effect β = .04 [−.01, .09], p = .086).
Figure 3.
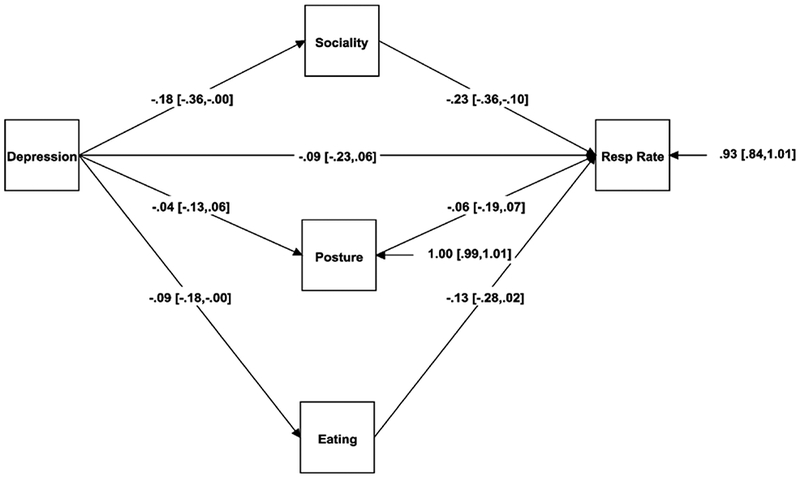
Mediation model for depression, social activity, and respiration rate.
Note. The regression coefficients are standardized; 95% confidence intervals are in brackets.
3.5.3. Anxiety
For anxiety, posture, eating, and physical activity were chosen to predict the five psychophysiological outcomes. No mediation was observed for any behavioral factors for anxiety.
4. Discussion
Previous psychophysiological research has largely emphasized examining the main effect of psychopathology on ANS functioning. Basic laboratory-based studies typically hold contextual variables (posture, activity, eating, drinking) constant via the study’s procedures and design. However, Houtveen and de Geus (2009) emphasize the importance of understanding how these non-psychological factors influence the association of psychopathology and psychophysiology. Thus, the present study explicitly examined the behavioral pathways between psychopathology and ANS functioning using ambulatory assessment.
By measuring impedance cardiography in daily life, the present preliminary study was able to examine the behavioral pathways between psychophysiology and depression, anxiety, and hypomanic personality. Rather than controlling for or holding constant behaviors, such as physical activity and social interaction, we were interested in how changes in everyday behaviors were affected by psychopathology and subsequently predicted sympathetic and parasympathetic ANS functioning. Ambulatory assessment, including experience sampling and ambulatory psychophysiology, is a powerful family of assessment tools for measuring dynamic real-world fluctuations in these constructs and for answering complex questions about the context of psychological findings.
We found that hypomanic personality was associated with increased reports of being physically active in the moment and that physical activity mediated the association between hypomanic personality, HRV, and HR. As participants became more physically active during the day, HR increased and HRV decreased. This finding has implications for thinking about discrepancies in the literature regarding the association of HRV and bipolar spectrum disorders. Previous research has been mixed as to whether bipolar spectrum disorders are associated with increased, decreased, or comparable HRV compared to healthy controls. Several studies have indicated elevated HRV (Gruber et al., 2015, 2011; Kang and Gruber, 2013), decreased HRV (Chang et al., 2014; Henry et al., 2010; Levy, 2014; Quintana et al., 2016), or no differences in HRV (Todder et al., 2005). Notably, all of these studies involved participants sitting still in a chair in the laboratory during testing. In thinking about how we characterize bipolar spectrum psychopathology, it is not surprising that physical activity is an important contextual variable to measure. Hypomanic personality, or bipolar spectrum psychopathology, is characterized by increased restlessness, increased energy, affective lability, and increased involvement in goal-directed activities (Kwapil et al., 2011; Sperry and Kwapil, 2017; Walsh et al., 2012). Thus, we might expect that as individuals experience more intense symptoms of mania or hypomania, they are likely going have increased physical activity, psychomotor agitation, or increased goal-directed activity, something that is constrained, to varying degrees, in laboratory experiments. These findings potentially lend support for theory that symptoms, or behaviors, of bipolar disorder and decreased parasympathetic nervous system functioning may share underlying neurobiological mechanisms. Future research should examine whether fronto-limbic functioning may play a role in both psychomotor activation and decreased HRV in bipolar disorder.
Social activity mediated the association of depressive symptoms and respiration rate: people who reported more severe symptoms of depression were less likely to be interacting with and around others (more likely to be alone). When with others, however, they were more likely to experience decreased respiration rate. This is consistent with our finding that being with others versus being alone was associated with decreased respiration. Respiration rate is not something that has attracted much attention in research on depression, so there’s no salient explanation anchored in past work; however, research has examined connections between social situations and ANS system functioning. Broadly speaking, the vagal nerve has been proposed as the intracranial nerve associated with emotional expression and communication in social settings, and low levels of RSA have subsequently been associated with emotional rigidity and poor social functioning (Carney et al., 2000; Rechlin et al., 2018). Furthermore, RSA has been more strongly or robustly associated with emotional processes in social contexts (Butler et al., 2006). While the present study found no associations between heart rate variability and depression, respiration is related to vagal control of the heart. The finding that those with depression display decreased respiration in social contexts is consistent with these findings; however, it is contrary to other findings that suggest that in general, increased respiration is usually found when communicating with others (Butler et al., 2006). Of note, studies that have seen respiration increases when communicating with others take into account time spent speaking with others, which the present study did not measure. Nevertheless, the finding is intriguing in light of the large literature on depression and HRV (Rottenberg, 2007), an outcome closely linked to respiratory dynamics (Grossman and Taylor, 2007).
Given past evidence for dysregulation of the ANS and anxiety, we were surprised to see that anxiety was not associated with any of the psychophysiological variables in daily life and that everyday behaviors did not mediate the association between anxiety and sympathetic and parasympathetic indices. It is important to note that there were relatively low levels of anxiety reported in this sample, which may be influencing these findings given that previous studies have reported both parasympathetic and sympathetic associations with trait anxiety symptoms outside of anxiety disorders (Miu et al., 2009). Furthermore, it is possible that in terms of mediation, the behaviors measured in this study (e.g., eating, drinking, posture) may have been less likely to be influenced by anxiety rather than depressive or hypomanic traits. However, anxiety was associated with eating and drinking in the moment. Alternatively, one interpretation is that the robust direct association of heart rate variability and anxiety found in a previous study (Miu et al., 2009; Thayer et al., 1996) reflects a more pathophysiological process and is less directly influenced by changes in behaviors than other forms of psychopathology.
4.1. Limitations and future directions
There were several limitations of the present study. First, this study was a preliminary examination of the behavioral mediation between depression, anxiety, and hypomanic traits with ANS functioning. Given its preliminary nature, the sample size of the study was likely underpowered to detect mediational effects. Thus, all significant findings should be interpreted with caution and need replication in larger sample sizes. Nevertheless, an important use of these results is to aid in forecasting likely effect sizes for future research.1
Second, due to the small sample size, we were unable compare alternative models that may have provided context for the relationships between psychopathology, behaviors, and ANS functioning. For example, the present study assumed that psychopathology predicted ANS functioning; however, some alternative theories, such as the polyvagal theory (Porges, 2001, 1995), explicitly theorize that ANS functioning predicts symptoms of psychopathology rather than the other direction. Alternative models could have disentangled reverse causation such that ANS dysregulation may have caused behaviors that in combination with emotions and cognitions, produce psychopathology. Future research should examine both potential models and compare fit.
Third, due to the limited sample size and low endorsement of behaviors of interest, potential interactions between different behaviors in predicting psychophysiology were not examined. For example, social activity and eating/drinking may have interacted to predict decreased respiration. Additionally, this study was primarily concerned with physical and social behaviors rather than intraindividual cognitive factors given evidence of their effect on ANS functioning. However, behaviors are inexplicably intertwined with cognitions. Future studies may examine where cognitive factors (e.g., rumination) may interact with behaviors to mediate psychopathology and ANS functioning.
Finally, future studies should examine the potential underlying neurobiological mechanisms that may be shared between everyday behaviors associated with psychopathology and ANS functioning such as front-limbic dopaminergic and noradrenergic pathways as well as cholinergic activity indicated in both depression and bipolar disorder. Longitudinal studies that involve both ecological assessments as well as neuroimaging studies will further disentangle the relationships between psychopathology, behaviors, and psychophysiology and the potential pathways by which they are connected.
4.2. Conclusions
The present study provided evidence that simultaneously measuring behavior and physiology using ambulatory assessments is worthwhile and may help elucidate mechanisms between behavior, psychopathology, and biology. Findings suggest that studies of psychophysiology could fruitfully examine, rather than hold constant or control for, everyday behaviors that are likely pathways from psychopathology symptoms to changes in parasympathetic and sympathetic ANS functioning.
Acknowledgments
This research was supported by the National Institute of Mental Health of the National Institutes of Health under award number R15MH079374. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Some of these findings were presented at the annual meeting the Society for Research on Psychopathology. We’re grateful to Roger Beaty, Ashley McHone, Zuzana Mironovová, and Emily Nusbaum for their assistance with this research.
Footnotes
Our sample size goal was to obtain at least 40 usable cases, which seemed reasonable given the preliminary nature of the study and the lack of a literature for estimating likely effect sizes. Power analysis is much more complex for two-level, clustered designs with unequal cluster sizes and extensive missingness—the sort found in experience sampling data, in which people vary greatly in how many surveys they complete (see Silvia, Kwapil, Walsh, & Myin-Germeys, 2014). Monte Carlo simulations were conducted using the observed relationship between hypomania and activity level (one of the larger effects of personality on behavioral mediators that we observed) as a “seed” effect size. Our simulations, conducted in Mplus 8, suggest that researchers should plan for at least 120 participants (with an average of 9 responses per participant) for power of around .80.
References
- Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP, 1998. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol. Assess 10, 176. [Google Scholar]
- Beauchaine T, 2001. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev. Psychopathol 13, 183–214. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen Y-J, Cacioppo JT, 2004. Where to Q in PEP. Psychophysiology 41, 333–337. 10.1111/j.1469-8986.2004.00156.x [DOI] [PubMed] [Google Scholar]
- Burns JW, Friedman R, Katkin ES, 1992. Anger Expression, Hostility, Anxiety, and Patterns of Cardiac Reactivity to Stress. Behav. Med 18, 71–78. 10.1080/08964289.1992.9935174 [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ, 2006. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology 43, 612–622. 10.1111/j.1469-8986.2006.00467.x [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS, 2000. Change in Heart Rate and Heart Rate Variability During Treatment for Depression in Patients With Coronary Heart Disease. Psychosom. Med 62. [DOI] [PubMed] [Google Scholar]
- Chang H-A, Chang C-C, Tzeng N-S, Kuo TBJ, Lu R-B, Huang S-Y, 2014. Heart rate variability in unmedicated patients with bipolar disorder in the manic phase. Psychiatry Clin. Neurosci 68, 674–682. 10.1111/pcn.12178 [DOI] [PubMed] [Google Scholar]
- Chen C-H, Suckling J, Lennox BR, Ooi C, Bullmore ET, 2011. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord 13, 1–15. 10.1111/j.1399-5618.2011.00893.x [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kotler M, Mittelman I, Osher Y, Bersudsky Y, 2003. Impaired heart rate variability in euthymic bipolar patients. Bipolar Disord 5, 138–143. 10.1034/j.1399-5618.2003.00027.x [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, 2014. Validity and Reliability of the Experience-Sampling Method, in: Flow and the Foundations of Positive Psychology: The Collected Works of Mihaly Csikszentmihalyi. Springer; Netherlands, Dordrecht, pp. 35–54. 10.1007/978-94-017-9088-8_3 [DOI] [Google Scholar]
- De Geus EJC, Van Doornen LJP, 1996. Ambulatory assessment of parasympathetic/sympathetic balance by impedance cardiography, in: Ambulatory Assessment: Computer-Assisted Psychological and Psychophysiological Methods in Monitoring and Field Studies. Hogrefe & Huber; Gottingen, Germany, pp. 141–163. [Google Scholar]
- Eckblad M, Chapman LJ, 1986. Development and validation of a scale for hypomanic personality. J. Abnorm. Psychol 95, 214. [DOI] [PubMed] [Google Scholar]
- Ernst JM, Litvack DA, Lozano DL, Cacioppo JT, Bernston GG, 1999. Impedance pneumography: Noise as signal in impedance cardiography. Psychophysiology 36, 333–338. https://doi.org/DOI: undefined [DOI] [PubMed] [Google Scholar]
- Fritz MS, MacKinnon DP, 2007. Required Sample Size to Detect the Mediated Effect. Psychol. Sci 18, 233–239. 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendolla GHE, Wright RA, Richter M, 2012. Effort intensity: Some insights from the cardiovascular system. Oxford Handb. Hum. Motiv 420–438. [Google Scholar]
- Glassman A, 2008. Depression and cardiovascular disease. Pharmacopsychiatry. 10.1055/s-2008-1058108 [DOI] [PubMed] [Google Scholar]
- Gorman JM, Sloan RP, 2000. Heart rate variability in depressive and anxiety disorders. Am. Heart J. 140, S77–S83. 10.1067/mhj.2000.109981 [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ, 2007. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30, 220–227. 10.1016/J.TINS.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW, 2007. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol 74, 263–285. 10.1016/J.BIOPSYCHO.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Purcell A, 2011. What goes up can come down? A preliminary investigation of emotion reactivity and emotion recovery in bipolar disorder. J. Affect. Disord 133, 457–466. 10.1016/j.jad.2011.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Mennin DS, Fields A, Purcell A, Murray G, 2015. Heart rate variability as a potential indicator of positive valence system disturbance: A proof of concept investigation. Int. J. Psychophysiol 98, 240–248. 10.1016/J.IJPSYCHO.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Paulus MP, Geyer MA, Perry W, 2010. Heart rate variability in bipolar mania and schizophrenia. J. Psychiatr. Res 44, 168–76. 10.1016/j.jpsychires.2009.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, 2005. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol 44, 227–239. 10.1348/014466505X29657 [DOI] [PubMed] [Google Scholar]
- Houtveen JH, de Geus EJC, 2009. Noninvasive Psychophysiological Ambulatory Recordings. Eur. Psychol 14, 132–141. 10.1027/1016-9040.14.2.132 [DOI] [Google Scholar]
- Houtveen JH, Groot PFC, De Geus EJC, 2005. Effects of variation in posture and respiration on RSA and pre-ejection period. Psychophysiology 42, 713–719. 10.1111/j.1469-8986.2005.00363.x [DOI] [PubMed] [Google Scholar]
- Kang Y, Gruber J, 2013. Harnessing happiness? Uncontrollable positive emotion in bipolar disorder, major depression, and healthy adults. Emotion. 10.1037/a0030780 [DOI] [PubMed] [Google Scholar]
- Kelsey RM, 2012. Beta-adrenergic cardiovascular reactivity and adaptation to stress: The cardiac pre-ejection period as an index of effort., in: How Motivation Affects Cardiovascular Response: Mechanisms and Applications. American Psychological Association, Washington, DC, US, pp. 43–60. 10.1037/13090-002 [DOI] [Google Scholar]
- Kelsey RM, Reiff S, Wiens S, Schneider TR, Mezzacappa ES, Guethlein W, 1998. The ensemble-averaged impedance cardiogram: An evaluation of scoring methods and interrater reliability. Psychophysiology 35, 337–340. https://doi.org/DOI: undefined [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM, 2010. Impact of Depression and Antidepressant Treatment on Heart Rate Variability: A Review and Meta-Analysis. Biol. Psychiatry 67, 1067–1074. 10.1016/j.biopsych.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Kogan A, Gruber J, Shallcross AJ, Ford BQ, Mauss IB, 2013. Too much of a good thing? Cardiac vagal tone’s nonlinear relationship with well-being. Emotion 13, 599. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Barrantes-Vidal N, Armistead MS, Hope GA, Brown LH, Silvia PJ, Myin-Germeys I, 2011. The expression of bipolar spectrum psychopathology in daily life. J. Affect. Disord 130, 166–70. 10.1016/j.jad.2010.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Miller MB, Zinser MC, Chapman LJ, Chapman J, Eckblad M, 2000. A longitudinal study of high scorers on the Hypomanic Personality Scale. J. Abnorm. Psychol 10.1037/0021-843X.109.2.222 [DOI] [PubMed] [Google Scholar]
- Levy B, 2014. Illness severity, trait anxiety, cognitive impairment and heart rate variability in bipolar disorder. Psychiatry Res 220, 890–5. 10.1016/j.psychres.2014.07.059 [DOI] [PubMed] [Google Scholar]
- Levy WC, Cerqueira MD, Harp GD, Johannessen K-A, Abrass IB, Schwartz RS, Stratton JR, 1998. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am. J. Cardiol 82, 1236–1241. 10.1016/S0002-9149(98)00611-0 [DOI] [PubMed] [Google Scholar]
- Licht CMM, Penninx BWJH, de Geus EJC, 2012. Effects of Antidepressants, but not Psychopathology, on Cardiac Sympathetic Control: A Longitudinal Study. Neuropsychopharmacology 37, 2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF, Lovibond SH, 1995. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther 33, 335–343. 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, Berntson GG, 2007. Where to B in dZ/dt. Psychophysiology 44, 113–119. 10.1111/j.1469-8986.2006.00468.x [DOI] [PubMed] [Google Scholar]
- McNeish D, Stapleton LM, Silverman RD, 2017. On the unnecessary ubiquity of hierarchical linear modeling. Psychol. Methods 22, 114. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Boesveldt S, Elbertse E, Berendse HW, 2008. Method to measure autonomic control of cardiac function using time interval parameters from impedance cardiography. Physiol. Meas 29, S383–S391. 10.1088/0967-3334/29/6/S32 [DOI] [PubMed] [Google Scholar]
- Miu AC, Heilman RM, Miclea M, 2009. Reduced heart rate variability and vagal tone in anxiety: trait versus state, and the effects of autogenic training. Auton. Neurosci 145, 99–103. 10.1016/j.autneu.2008.11.010 [DOI] [PubMed] [Google Scholar]
- Model 50–2303-00. (2014). Gahannah, OH: Mindware Technologies. [Google Scholar]
- Moon E, Lee S-H, Kim D-H, Hwang B, 2013. Comparative Study of Heart Rate Variability in Patients with Schizophrenia, Bipolar Disorder, Post-traumatic Stress Disorder, or Major Depressive Disorder. Clin. Psychopharmacol. Neurosci 11, 137–143. 10.9758/cpn.2013.11.3.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist PA, 1976. The Cardiovascular-Behavioral Interaction?As It Appears Today. Psychophysiology 13, 95–107. 10.1111/j.1469-8986.1976.tb00081.x [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D, 2009. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion 9, 265. [DOI] [PubMed] [Google Scholar]
- Parker G, 2000. Classifying Depression: Should Paradigms Lost Be Regained? Am. J. Psychiatry 157, 1195–1203. 10.1176/appi.ajp.157.8.1195 [DOI] [PubMed] [Google Scholar]
- Penninx BWJH, Beekman A, Honig A, Deeg D, Schoevers R, van Eijk J, van Tilburg W, 2001. Depression and cardiac mortality: Results from a community-based longitudinal study. Arch. Gen. Psychiatry 58, 221–227. [DOI] [PubMed] [Google Scholar]
- Porges SW, 2001. The polyvagal theory: phylogenetic substrates of a social nervous system. Int. J. Psychophysiol 42, 123–146. 10.1016/S0167-8760(01)00162-3 [DOI] [PubMed] [Google Scholar]
- Porges SW, 1995. Cardiac vagal tone: A physiological index of stress. Neurosci. Biobehav. Rev 19, 225–233. 10.1016/0149-7634(94)00066-A [DOI] [PubMed] [Google Scholar]
- Quintana DS, Westlye LT, Kaufmann T, Rustan ØG, Brandt CL, Haatveit B, Steen NE, Andreassen OA, 2016. Reduced heart rate variability in schizophrenia and bipolar disorder compared to healthy controls. Acta Psychiatr. Scand 133, 44–52. 10.1111/acps.12498 [DOI] [PubMed] [Google Scholar]
- Rechlin T, Weis M, Spitzer A, Kaschka WP, 2018. Are affective disorders associated with alterations of heart rate variability? J. Affect. Disord 32, 271–275. 10.1016/0165-0327(94)90091-4 [DOI] [PubMed] [Google Scholar]
- Reilly KJ, Moore CA, 2003. Respiratory Sinus Arrhythmia During Speech Production. J. Speech, Lang. Hear. Res 46, 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie KL, Hemingway H, Kumari M, Brunner E, Malik M, Marmot M, 2003. Effects of Moderate and Vigorous Physical Activity on Heart Rate Variability in a British Study of Civil Servants. Am. J. Epidemiol 158, 135–143. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, 2007. Cardiac vagal control in depression: A critical analysis. Biol. Psychol 74, 200–211. 10.1016/J.BIOPSYCHO.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Schaffer F, Ginsberg JP, 2017. An overview of heart rate variability metrics and norms. Front Public Health. 5, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Beaty RE, Nusbaum EC, Eddington KM, Kwapil TR, 2014a. Creative motivation: Creative achievement predicts cardiac autonomic markers of effort during divergent thinking. Biol. Psychol 102, 30–37. 10.1016/J.BIOPSYCHO.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Eddington KM, Beaty RE, Nusbaum EC, Kwapil TR, 2013. Gritty people try harder: Grit and effort-related cardiac autonomic activity during an active coping challenge. Int. J. Psychophysiol 88, 200–205. 10.1016/J.IJPSYCHO.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Nusbaum EC, Eddington KM, Beaty RE, Kwapil TR, 2014b. Effort deficits and depression: The influence of anhedonic depressive symptoms on cardiac autonomic activity during a mental challenge. Motiv. Emot 38, 779–789. 10.1007/s11031-014-9443-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrondal A, Rabe-Hesketh S, 2004. Generalized latent variable modeling: Multilevel, longitudinal, and structural equation models. Crc Press. [Google Scholar]
- Sperry SH, Kwapil TR, 2017. What can daily life assessment tell us about the bipolar spectrum? Psychiatry Res 252, 51–56. 10.1016/j.psychres.2017.02.045 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD, 1996. Autonomic characteristics of generalized anxiety disorder and worry. Biol. Psychiatry 39, 255–266. 10.1016/0006-3223(95)00136-0 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2009. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev 33, 81–88. 10.1016/J.NEUBIOREV.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2000. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord 61, 201–16. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Smith M, Rossy LA, Sollers JJ, Friedman BH, 1998. Heart period variability and depressive symptoms: gender differences. Biol. Psychiatry 44, 304–306. 10.1016/S0006-3223(98)00008-0 [DOI] [PubMed] [Google Scholar]
- Todder D, Bersudsky Y, Cohen H, 2005. Nonlinear analysis of RR interval in euthymic bipolar disorder. Auton. Neurosci 117, 127–31. 10.1016/j.autneu.2004.11.006 [DOI] [PubMed] [Google Scholar]
- van Eekelen APJ, Houtveen JH, Kerkhof GA, 2004. Circadian variation in base rate measures of cardiac autonomic activity. Eur. J. Appl. Physiol 93, 39–46. 10.1007/s00421-004-1158-6 [DOI] [PubMed] [Google Scholar]
- van Eijnatten MAJM, van Rijssel MJ, Peters RJA, Verdaasdonk RM, Meijer JH, 2014. Comparison of cardiac time intervals between echocardiography and impedance cardiography at various heart rates. J. Electr. Bioimpedance; Vol 5. [Google Scholar]
- van Lien R, Schutte NM, Meijer JH, De Geus EJC, 2013. Estimated preejection period (PEP) based on the detection of the R-wave and dZ/dt-min peaks does not adequately reflect the actual PEP across a wide range of laboratory and ambulatory conditions. Int. J. Psychophysiol 87, 60–69. 10.1016/J.IJPSYCHO.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Volkers AC, Tulen JHM, van den Broek WW, Bruijn JA, Passchier J, Pepplinkhuizen L, 2003. Motor activity and autonomic cardiac functioning in major depressive disorder. J. Affect. Disord 76, 23–30. 10.1016/S0165-0327(02)00066-6 [DOI] [PubMed] [Google Scholar]
- Walsh MA, DeGeorge DP, Barrantes-Vidal N, Kwapil TR, 2015. A 3-year longitudinal study of risk for bipolar spectrum psychopathology. J. Abnorm. Psychol 10.1037/abn0000045 [DOI] [PubMed] [Google Scholar]
- Walsh MA, Royal A, Brown LH, Barrantes-Vidal N, Kwapil TR, 2012. Looking for bipolar spectrum psychopathology: identification and expression in daily life. Compr. Psychiatry 53, 409–21. 10.1016/j.comppsych.2011.06.006 [DOI] [PubMed] [Google Scholar]
- Weber CM, Smith A, 1990. Autonomic Correlates of Stuttering and Speech Assessed in a Range of Experimental Tasks. J. Speech, Lang. Hear. Res 33, 690–706. [DOI] [PubMed] [Google Scholar]


