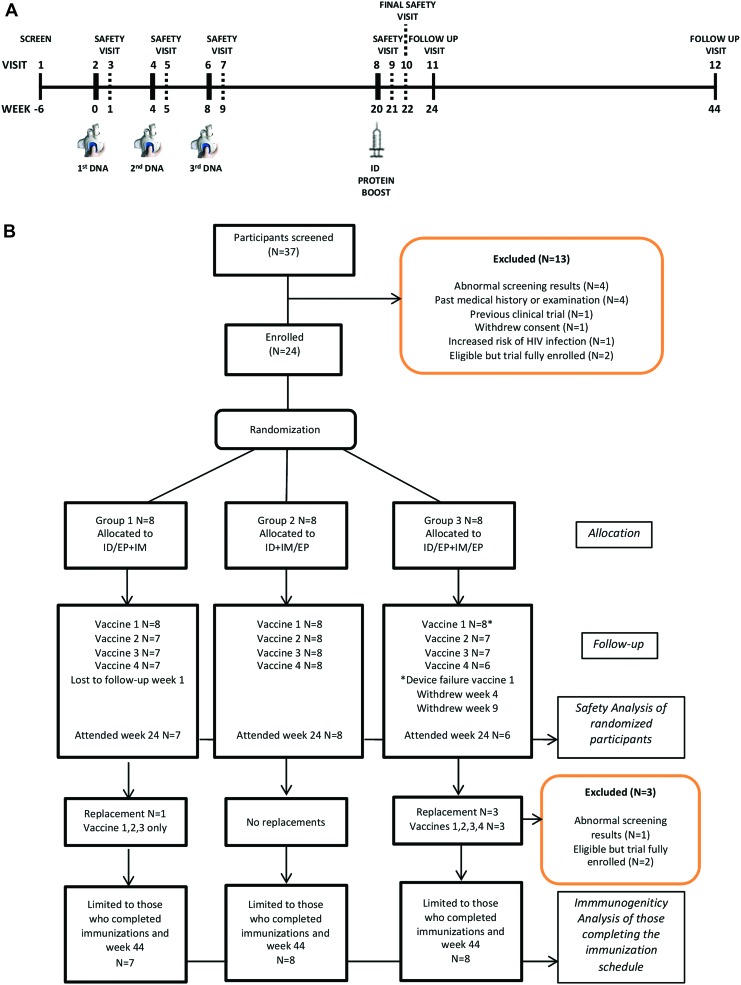
Figure 1.
(A) Trial schematic. Participants attended a total of 12 visits, with DNA immunizations given at week 0 (visit 2), week 4 (visit 4), and week 8 (visit 6) followed by an intradermal (i.d.) booster injection of recombinant CN54gp140 at week 20 (visit 8). Blood was taken from participants at each visit, and mucosal samples were taken at weeks 0, 20, and 22 (visits 2, 8, and 10, respectively). Thick solid lines indicate an immunization visit (weeks 0, 4, 8, and 20). Dotted lines indicate a safety visit (weeks 1, 5, 9, 21, and 22). Thin solid lines indicate a screen or follow-up visit (weeks 6, 24, and 44). (B) CONSORT flow diagram. Participants were randomized into one of three groups. The numbers of participants enrolled, randomized, followed up, and analyzed are shown for each treatment group. Color images available online at www.liebertpub.com/hum