Summary
Background
Poor breast cancer survival in low-income and middle-income countries (LMICs) can be attributed to advanced-stage presentation and poor access to systemic therapy. We aimed to estimate the outcomes of different early detection strategies in combination with systemic chemotherapy and endocrine therapy in LMICs.
Methods
We adapted a microsimulation model to project outcomes of three early detection strategies alone or in combination with three systemic treatment programmes beyond standard of care (programme A): programme B was endocrine therapy for all oestrogen-receptor (ER)-positive cases; programme C was programme B plus chemotherapy for ER-negative cases; programme D was programme C plus chemotherapy for advanced ER-positive cases. The main outcomes were reductions in breast cancer-related mortality and lives saved per 100 000 women relative to the standard of care for women aged 30–49 years in a low-income setting (East Africa; using incidence data and life tables from Uganda and data on tumour characteristics from various East African countries) and for women aged 50–69 years in a middle-income setting (Colombia).
Findings
In the East African setting, relative mortality reductions were 8–41%, corresponding to 23 (95% uncertainty interval −12 to 49) to 114 (80 to 138) lives saved per 100 000 women over 10 years. In Colombia, mortality reductions were 7–25%, corresponding to 32 (−29 to 70) to 105 (61 to 141) lives saved per 100 000 women over 10 years.
Interpretation
The best projected outcomes were in settings where access to both early detection and adjuvant therapy is improved. Even in the absence of mammographic screening, improvements in detection can provide substantial benefit in settings where advanced-stage presentation is common.
Introduction
In the USA, breast cancer death rates decreased by 36% between 1989 and 2012.1 These improvements were driven by combined improvements in early detection and adjuvant systemic therapy.2
Women in low-income and middle-income countries (LMICs) face various barriers to breast cancer care, from accessing early detection programmes to receiving timely diagnosis and appropriate treatment. This situation is reflected in breast cancer 5-year survival outcomes, which are 40–60% in LMICs versus 84% in North America.3
Scarce health-care resources in LMICs need to be used strategically to ensure timely, beneficial access to breast health care. Evidence-based, resource appropriate guidelines, such as the Breast Health Global Initiative’s resource-stratified guidelines4–6 and the National Comprehensive Cancer Network’s resource-stratified framework for breast cancer treatment,7 provide broad direction for the allocation of resources. However, to make optimal decisions in a given clinical setting, policy makers have to weigh expected benefits of feasible strategies against implementation costs. We have developed a simple and transparent evidence-based microsimulation model to project outcomes of interventions for breast cancer screening and treatment, with minimal assumptions about the natural history of the disease.8 A key feature of this model is that it decouples the effect of early detection from that of treatment in the prediction of changes in survival. This separation allows for the assessment of how changes in availability of systemic therapies for breast cancer might affect screening efficacy in the context of historical mammographic screening trials.9
In this study, we aimed to investigate the effect of improved screening and treatment for breast cancer in a low-income setting (the East African region) and an upper middle-income setting (Colombia) by inputting systemic therapies and early detection strategies into our existing model. We also introduce an accessible online interface that allows users to project the outcomes for customised settings, using inputs based on region-specific data.
Methods
Model structure
The modelling framework we used has already been described in detail.8 We used a microsimulation approach to model individual-level state transitions from healthy to clinically diagnosed breast cancer and, finally, death, for a cohort of women with a specified age distribution (30–49 years or 50–69 years) at the start of the study (figure 1). This model yields a set of breast cancer records for a hypothetical population. We specified the age, stage at diagnosis (early is stages I to II, advanced is stages III to IV), and oestrogen-receptor (ER) status (ER-positive or ER-negative) of the fraction of women from the simulated cohort who have breast cancer. These features represent a minimal set of major prognostic factors for the assessment of systemic treatment planning and breast cancer survival outcomes. Baseline disease-specific survival (survival in the absence of treatments modelled in the policy intervention) is longer for early stage cases than it is for advanced-stage cases. These stage-specific survival data should be disease-specific (ie, in the absence of death from another cause). When survival data include known dissemination of systemic treatments, which was true for some of our survival datapoints, a mathematical conversion to baseline survival can be applied, as shown in the appendix. Policy interventions can change indicated treatments and detect cases of breast cancer early. Treatment extends survival beyond the baseline. We model early detection interventions as shifting some cases that would have been diagnosed with advanced-stage disease to an earlier stage at diagnosis, which improves their survival after clinical diagnosis.10,11 This shift in disease stage has a cascade effect on treatment, since advanced-stage cases often require more extensive systemic therapy than do early stage diagnoses. For example, ER-positive and node-positive breast cancers usually warrant the use of both cytotoxic chemotherapy and endocrine therapy, whereas ER-positive and node-negative cases often respond to endocrine therapy alone.12 Thus, early detection strategies downstage disease and reduce the extent of required systemic treatment, which are both highly relevant for planning cancer control in LMICs.
Figure 1: Schematic of the state-transition microsimulation model.
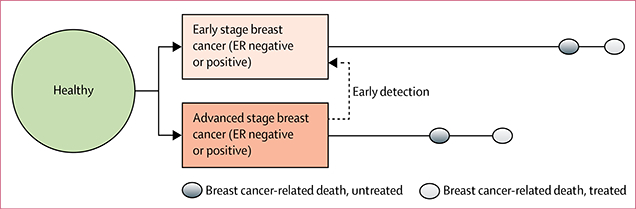
Dashed arrow shows that early detection interventions decrease advanced-stage incidence. ER=oestrogen-receptor.
Although early detection programmes will cause breast cancer incidence to increase, the primary determinant of mortality from breast cancer in a given population will be cases that would have been identified before these early detection efforts began. Thus, we modelled the same number of cases with and without early detection, and always projected survival from their original date of clinical diagnosis without screening. Treatments were combinations of adjuvant chemotherapies and tamoxifen (endocrine therapy), with efficacies based on international meta-analyses of randomised trials.13,14 Although chemotherapy is used to treat both early and advanced-stage ER-negative cancers and advanced (node-positive) ER-positive cancers,12 endocrine therapy is only used to treat ER-positive tumours. These systemic therapies extend survival for early and advanced-stage disease.
Stage distribution under early detection
We defined three scenarios for cancer stage distribution. Each successive scenario represents a reduction in advanced-stage diagnosis, reflected in decreased incidence of advanced-stage cancer. As the true stage distribution under these proposed scenarios is unknown in many settings, we used data from known sources. All stage distributions refer to a reference population in the absence of early detection.
The first scenario was poor awareness. Advanced-stage breast cancer is common in settings with no screening and low public awareness of breast cancer as a health issue—for example, in the USA during the 1930s and 1940s. The Connecticut Tumor Registry15 shows that 60% of patients with cancer were diagnosed in the advanced stage in 1935–1941. Thus, unless country-specific stage distributions are available, our level 1 scenario assumes that in countries and regions without screening or early detection programmes, and with low public awareness, 60% of breast cancers will present as advanced-stage disease.
The second scenario was elevated breast cancer awareness and clinical screening. When public awareness of breast cancer early detection is high, women are more likely to present for evaluation with smaller masses that can be clinically diagnosed on the basis of history and clinical breast examination. The Canadian National Breast Screening Study16–18 randomly assigned women, between 1980 and 1985, to an intervention group (mammographic screening) or to a control group (clinical evaluation), including clinical breast examination for 5 years, breast cancer awareness, and clinical screening. Since 35% of patients in the control group had advanced-stage disease, our model assumes that, in a setting where breast health awareness is prevalent and clinical access is available but mammographic screening is not, 35% of patients with breast cancer will present as advanced-stage disease.
The third scenario was mammographic screening, which reduces the incidence of presentation of advanced-stage disease by an estimated 15%.19 By applying this relative reduction to the 35% advanced-stage in the second scenario, our model assumes that, in a country where awareness of breast health is prevalent and mammographic screening is available, 30% of patients with breast cancer will present as advanced-stage disease.
Cancer treatment modelling
Our model assumes that all patients receive surgical therapy with or without radiation treatment for locoregional control, as reflected by cancer survival outcomes in the specific locations we modelled. To address the potential effects of improved programmes of systemic therapy, we modelled three scenarios beyond the standard of care. Programme A was current standard of care. Programme B was endocrine (tamoxifen or aromatase inhibitor) therapy for all ER-positive cases, including both early and advanced-stage disease. Programme C was programme B plus (anthracycline-based) chemotherapy for all ER-negative cases. Programme D was programme C plus chemotherapy for advanced (node positive) ER-positive cases.
Model inputs
In sub-Saharan East Africa, breast cancer cases commonly present in the advanced stages.20 The underlying causes include patient delays in seeking care and inability of primary care health-care providers to recognise early stage tumours; more than 40% of cases in the East African region have delayed diagnosis (>12 months from symptomatic presentation to diagnosis).21 Although patients with cancer undergo surgical resection, standard drug therapy (endocrine or chemotherapy) is not consistently available.
The Colombian population receives medical care through four different health plans: general health insurance for employees and their families, subsidised health insurance for low-income individuals, public hospital assistance for people without any health insurance, and prepaid insurance for high-income individuals.22 Each plan is managed by insurance companies that provide health services via a network of clinics.22 Surgery, radiation, and systemic chemotherapy and endocrine therapy are available, but are not uniformly accessible. Opportunistic mammographic screening is available and has been endorsed for women aged 50–69 years; however, women within this age group are commonly not referred for screening. Thus, advanced-stage presentation is relatively common in Colombia.23
We used various parameters and values to model the standard of care in the East African region (low-income) and Colombia (upper middle-income; table 1). Location-specific and age-specific incidences were from the Cancer Incidence in Five Continents database.24 We used data from Uganda to represent the East African region. Stage distributions, ER status, and survival data were from location-specific literature. Since some baseline survival estimates include a proportion of the population receiving systemic treatment, we used a mathematical conversion to quantify baseline survival estimates in the absence of these treatments (appendix).
Table 1:
Inputs to the microsimulation model by seven parameters
| Measures | East African region | Colombia | |
|---|---|---|---|
| 1 | Clinical breast cancer incidence, by age | CI5-X,24 Rakai, Uganda (2003–07) | CI5-X,24 Cali, Colombia (2003–07) |
| 2* | Proportion of cases that are advanced stage (stage III or IV) at clinical diagnosis (%) |
78% (meta-analysis of studies from various countries in sub-Saharan Africa)20 |
45%25 |
| 3 | Proportion of ER-positive cases (%) | 41% (Kenya, Uganda, Tanzania, and Madagascar)26 | 70%27 |
| 4* | Modelled proportion of the population receiving each available treatment (A-D), by stage at diagnosis and tumour type (%) |
A: surgery only; B: A plus endocrine therapy for 100% of ER- positive cases; C: B plus chemotherapy for 100% of ER-negative cases; and D: C plus chemotherapy for 100% of advanced-stage ER-positive cases |
A: surgery plus endocrine therapy and chemotherapy;† B: A plus endocrine therapy for 100% of ER-positive cases; C: B plus chemotherapy for 100% of ER-negative cases; and D: C plus chemotherapy for 100% of advanced-stage ER-positive cases |
| 5 | Treatment efficacies (ie, reduction in the risk of disease-specific mortality from each treatment), by stage at diagnosis |
Endocrine therapy: HR=0∙70 for ER-positive cases, 1∙0 for ER-negative cases; chemotherapy: HR=0∙775 for all; endocrine therapy plus chemotherapy: HR=0∙5425 for ER-positive cases, 0∙775 for ER-negative cases (meta-analyses of treatment trials worldwide, primarily USA, Europe, and Japan) 13,14 |
Same as for East Africa |
| 6 | Baseline disease-specific survival (in the absence of systemic treatment), by stage at diagnosis‡ |
Advanced 35% (Uganda),28 early 69% (Ethiopia)29 | Advanced 41%,30 early 84%30 |
| 7 | Other-cause mortality rates, by age | IHME lifetables, Uganda 201331 | IHME lifetables, Colombia 201331 |
In the East African region, we modelled ten policy interventions (E0-E9) for women aged 30–49 years (table 2). 78% of patients with breast cancer in this region present with advanced-stage disease.20 This proportion of advanced-stage distribution is substantially higher than the 60% advanced-stage rate in the Connecticut Tumor Registry from 1935 to 1941,15 suggesting that many people with early stage disease are not accessing health care to be diagnosed and counted. The efficacy of organised screening depends on all patients having access to care and more than 70% of the population being screened,33 which is not feasible in the East African region. Therefore, we did not include mammographic detection scenarios in our models. Since we assumed that endocrine treatment and chemotherapy are not typically available, we modelled treatment programmes B to D without any correction for drug therapy.
Table 2:
10-year cumulative breast cancer outcomes in the East African region, by early detection strategy
| No early detection (78% advanced) |
Clinical access only (60% advanced) |
Elevated awareness with clinical screening (35% advanced) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Policy E0 | Policy E1 | Policy E2 | Policy E3 | Policy E4 | Policy E5 | Policy E6 | Policy E7 | Policy E8 | Policy E9 | |
| Treatment programme |
A | B | C | D | B | C | D | B | C | D |
| Incidence | 616 (563–663) | 616 (563–663) | 616 (563–663) | 616 (563–663) | 616 (563–663) | 616 (563–663) | 616 (563–663) | 616 (563–663) | 616 (563–663) | 616 (563–663) |
| Mortality | 277 (244–303) | 254 (227–281) | 231 (203–265) | 217 (190–247) | 226 (196–253) | 206 (178–242) | 195 (167–224) | 190 (167–213) | 171 (146–198) | 164 (139–186) |
| Proportion of incident survival (%) |
55∙0% (51∙6–59∙6) |
58∙8% (55∙2–62∙3) |
62∙6% (58–8–66–3) |
64–8% (60–6–68–2) |
63–3% (59–8–66–8) |
66–6% (63–1–70–5) |
68–4% (64–1–71–1) |
69–2% (66–1–72–1) |
72–2% (68–6–75–5) |
73–5% (69–5–76–6) |
| MRR | 1 | 0∙92 (0∙83–1∙05) |
0∙83 (0–74–0–93) |
0–78 (0–69–0–89) |
0–82 (0–72–0–92) |
0–74 (0–66–0–84) |
0–70 (0–61–0–80) |
0–69 (0–61–0–79) |
0–62 (0–54–0–71) |
0–59 (0–51–0–68) |
| ARR | 0 | 23 (−12 to 49) |
46 (16 to 74) |
60 (30 to 90) |
51 (19 to 80) |
71 (42 to 98) |
82 (53 to 12) |
87 (53 to 115) |
106 (74 to 132) |
113 (80 to 138) |
| YLS | 0 | 92 (−12 to 200) |
179 (64 to 298) |
227 (85 to 335) |
191 (70 to 312) |
268 (177 to 380) |
305 (165 to 421) |
324 (209 to 438) |
395 (280 to 523) |
418 (282 to 552) |
Data are mean (95% uncertainty intervals) from 100 simulations of 100 000 women aged 30–49 years. MRR, ARR, and YLS are relative to policy E0. A=surgery only. B=A plus endocrine therapy for all ER-positive cases. C=B plus chemotherapy for all ER-negative cases. D=C plus chemotherapy for advanced ER-positive cases. ER=oestrogen-receptor. MRR=mortality rate ratio. ARR=absolute risk reduction (ie, number of lives =years of lite saved).
In Colombia, we also modelled ten policy Interventions (C0-C9) for women aged 50–69 years and included mammographic detection scenarios (table 3). Better access to health care and awareness of breast health is reflected in the contemporary Colombian stage distribution of 45% advanced-stage disease.25 Since this proportion is already an improvement from the 60% advanced-stage distribution seen in the Connecticut registry, we only modelled levels 2 and 3. As an upper middle-income country, Colombia has an effective health-care system in which patients with insurance have access to standard drug therapy. In our model, we applied treatment frequencies for breast cancer in the USA in 2000 (table 3; appendix),32 but we recognise that this method might overestimate actual treatment in Colombia nowadays.
Table 3:
10-year cumulative breast cancer outcomes in Colombia, by early detection strategy
| No early detection (45% advanced) |
Elevated awareness with clinical screening (35% advanced) |
Mammographic screening (30% advanced) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Policy C0 | Policy C1 | Policy C2 | Policy C3 | Policy C4 | Policy C5 | Policy C6 | Policy C7 | Policy C8 | Policy C9 | |
| Treatment programme |
A* | B | C | D | B | C | D | B | C | D |
| Incidence | 1767 (1691–1854) |
1767 (1691–1854) |
1767 (1691–1854) |
1767 (1691–1854) |
1767 (1691–1854) |
1767 (1691–1854) |
1767 (1691–1854) |
1767 (1691–1854) |
1767 (1691–1854) |
1767 (1691–1854) |
| Mortality | 424 (377–456) |
393 (355–437) |
394 (360–432) |
388 (351–422) |
347 (316–386) |
348 (313–385) |
343 (306–375) |
325 (292–362) |
325 (293–359) |
319 (289–356) |
| Proportion of incident survival (%) |
76–0% (74–5–78–4) |
77–8% (75–7–79–7) |
77–7% (76–1–79–7) |
78–0% (76–5–79–7) |
80–4% (78–3–82–1) |
80–3% (78–4–82–5) |
80–6% (79–0–82–1) |
81–6% (79–8–83–4) |
81–6% (80–0–83–3) |
81–9% (79–9–83–4) |
| MRR | 1 | 0–93 (0–84–1–07) |
0–93 (0–83–1–03) |
0–92 (0–83–1–02) |
0–82 (0–73–0–95) |
0–82 (0–75–0–91) |
0–81 (0–73–0–90) |
0–77 (0–69–0–90) |
0–77 (0–69–0–88) |
0–75 (0–68–0–85) |
| ARR | 0 | 31 (−29 to 70) |
30 (−11 to 70) |
36 (−6 to 73) |
77 (19 to 122) |
76 (34 to 111) |
81 (38 to 118) |
99 (39 to 135) |
100 (47 to 135) |
105 (61 to 141) |
| YLS | 0 | 122 (−68 to 302) |
116 (−100 to 307) |
139 (−53 to 308) |
286 (100 to 462) |
283 (46 to 468) |
304 (96 to 463) |
367 (152 to 548) |
371 (128 to 574) |
387 (183 to 551) |
Data are mean (95% uncertainty intervals) from 100 simulations of 100 000 women aged 50–69 years. MRR, ARR, and YLS are relative to policy C0. A=surgery only plus some endocrine therapy or chemotherapy, or both, for some cases. B=A plus endocrine therapy for all ER-positive cases. C=B plus chemotherapy for all ER-negative cases. D=C plus chemotherapy for advanced ER-positive cases. ER=oestrogen-receptor. MRR=mortality rate ratio. ARR=absolute risk reduction (ie, number of lives saved). YLS=years of life saved. *Standard of care use of endocrine therapy or chemotherapy was approximated from distributions in the USA in 2000 (68% of ER-positive cases receive at least endocrine therapy and 73% of ER-negative cases receive at least chemotherapy; appendix).32
Our model can accommodate settings in which a proportion of the population is already receiving the treatment of interest; in this case, we model interventions that extend access to that treatment. The overall disease-specific survival, the proportion receiving treatment, and the efficacy of treatment on baseline survival must be known. The appendix shows how this information can be used to recover an estimate of baseline survival (in the absence of treatment), which can then be entered as an input into the model along with the specified treatment target.
Model outcomes
For both the standard-of-care and intervention scenarios, the model projects cumulative breast cancer incidence (C), death counts (D), incident survival (%; 100 × (C-D)/C), and years lived (Y) among the cohort over the given follow-up interval. To quantify the benefit of an intervention, we compared the intervention setting (I) to the standard-of-care (S) using three statistics: mortality rate ratio (DI/DS, where D is the death count; measure of relative reduction in mortality), absolute risk reduction (DS-DI; measure of absolute number of lives saved), and years of life saved (YLS; YS-YI, where Y is the years lived). Absolute risk reduction and YLS are highly sensitive to the specified follow-up interval and generally increase with time. Uncertainty intervals are derived by running repeated simulation models with constant parameter inputs and varying the random number seed.
Model access and user interface
Our model code is available online, with a user-friendly interactive web interface to run the model. Users can customise inputs for various LMICs and early detection or treatment interventions. Although parameters 2–7 (table 1) must be specified from the literature, the interface provides regional incidence and all-cause mortality data via incidence24 and lifetable31 databases. We developed the model and interface using R (version 3.3.0) and the RShiny platform (shiny 1.0.0).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We report 10-year cumulative breast cancer outcomes projected after the introduction of each of the modelled policy interventions in the East African region (E0-E9; table 2) and Colombia (C0-C9; table 3), where E0 and C0 approximate the current standard-of-care in each location.
For the 616 incident cases in women aged 30–49 years in the East African region case scenario, the absolute risk reduction (ie, lives saved) ranges from 23 (95% uncertainty interval [UI] −12 to 49; policy E1: no early detection efforts, tamoxifen for all ER-positive cases) to 113 (80 to 138; policy E9: clinical breast examination plus tamoxifen for all ER-positive cases, chemotherapy for all ER-negative and advanced ER-positive cases). This finding translates to 92–418 YLS and a mortality rate ratio of 0·59 to 0·92—ie, relative mortality reductions of 8–41% (table 2). Mortality projections for interventions E6 and E7 show that different combinations of treatment and early detection efforts might lead to similar increases in survival. We project that programme D, the more aggressive treatment with only breast education (E6), will reduce mortality to the same extent as programme B, the less aggressive treatment paired with clinical breast examination (E7), will.
Treatment benefit is greatest in the absence of early detection (figure 2A). Progression from treatment programme B to D with no early detection strategy (interventions E1-E3) saves between 23 and 60 lives. That same treatment progression under clinical breast examination (policies E7-E9) saves between 87 and 114 lives. The final treatment progression (interventions E8-E9) saves an additional eight lives. This finding shows the interaction of early detection and treatment in our model. As early detection improves, the incremental benefit of treatment decreases because, with favourable baseline survival, there are fewer lives to be saved.
Figure 2: Absolute risk reduction for each intervention compared with the standard of care in (A) the East African region and (B) Colombia.
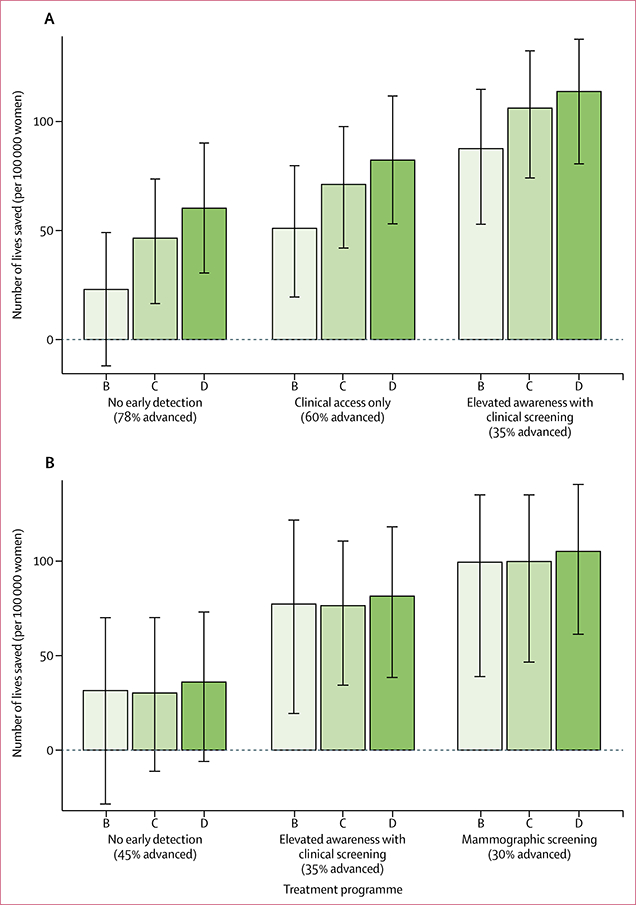
Error bars are 95% uncertainty intervals. Percentages in brackets are the proportions of women who present with advanced-stage disease. ER=oestrogen-receptor. B=endocrine therapy for ER-positive cases. C=B plus chemotherapy for ER-negative cases. D=C plus chemotherapy for advanced ER-positive cases.
We project 1767 incident cases in women aged 50–69 years in Colombia with absolute gains in lives saved ranging from 31 (95% UI −29 to 70; C1: tamoxifen for all ER-positive cases) to 105 (61 to 141; C9: mammographic screening plus endocrine therapy for all ER-positive cases, chemotherapy for all ER-negative cases, and chemotherapy for advanced ER-positive cases; table 3), which is similar to those in the East African region (table 2). The older age at intervention limits the potential for YLS, which range from 122 years to 387 years. The results also reflect the better baseline stage distribution and survival in Colombia (table 1), which we attribute to the presence of some education on breast cancer. Relative gains range from mortality rate ratios of 0·75 to 0·93—ie, mortality reductions of 7–25% (table 3).
In Colombia, the incremental benefits of successive treatment with programmes C and D are negligible with all types of early detection (figure 2B). This finding reflects the good access to systemic therapy under the current standard of care (table 1).
Uncertainty intervals are wide in both settings because of the relatively small number of incident cases per 100 000 women (figure 2, tables 2, 3). Some of the treatment-only interventions (E1 and C1-C3) might not represent clinically significant improvements over the current standard of care because such policies only benefit a small proportion of cases in both settings—ie, all ER-positive cases in the East African region and the untreated ER-positive and ER-negative cases in Colombia.
Discussion
This modelling framework quantifies the effect of breast cancer control efforts on mortality in low-income and middle-income settings and shows that different combinations of treatment and early detection interventions can yield similar mortality reductions in a given setting. This evidence can be used to optimise resource allocation. Our model is accessible via a user-friendly interface that allows non-experts to select data inputs that best approximate standard of care, and allows them to project a range of outcomes that are relevant to their preferred options for disease control.
Modelling is often used to develop policy for the control of cancer, allowing the synthesis of available information to fill in the gaps in evidence that remain when empirical investigations—such as randomised controlled trials— are not feasible. This situation is especially relevant in LMICs; without successful breast cancer control efforts, 65% of breast cancer-related deaths will occur in LMICs by 2025.34 Many models of breast cancer progression, detection, and outcomes exist,35–38 including several that are specific to LMICs.39,40 Our model differs from these in that it does not include the natural history of the underlying disease. Instead, it focuses on observable events in disease progression, which requires relatively few inputs from users, making the model highly transparent and portable across applications. Our modelling framework can be used by policy makers across various settings without the need for customisation for each new application.
Given the scarcity of available resources in LMICs, pragmatic planning—at least in the short term—is required to use few resources to benefit the largest proportion of the population. Easy-to-use models that can evaluate the performance of feasible strategies will be useful in LMICs. Our examples quantify the potential benefit of early detection and diagnosis strategies alone and in combination with endocrine and systemic chemotherapies. We anticipate that users of the model will focus on affordable and practical policies in their local setting; therefore, we focus on benefits rather than costs.
Affordability is dependent upon the characteristics of the health-care system and known constraints to implementation. In the East African region, the burden of health-care costs falls primarily on patients. Low amounts of health spending have resulted in dysfunctional referral systems and fragmented health services, which contribute to advanced-stage diagnoses and poor outcomes. Policies that are relevant to such low-resource settings should be implemented with a resource-stratified, phased implementation approach. For example, a series of recommendations for Tanzania prioritises detection, treatment, and diagnosis of symptomatic breast cancers in primary care via the evaluation of symptoms through clinical breast examination. Additionally, educational activities are recommended to increase awareness of the importance of seeking care for breast cancer symptoms.
A review of Colombian breast health care reported that, although opportunistic mammographic screening is covered by contributory and subsidised health insurance plans, it is not organised well. A model to address implementation-related challenges in this setting was reported in a Peruvian study that involved stakeholders from the Peruvian Ministry of Health, the National and Regional Cancer Institutes, and non-governmental organisations. The intervention successfully targeted all aspects of breast health care by improving awareness of breast cancer, introducing resource appropriate early diagnostic technologies in local clinics, and building a successful referral system to regional cancer centres for locoregional and systemic treatment.
Perhaps the greatest challenge from a modelling perspective is the model’s dependence on inputs that are not routinely collected by registries in LMICs, including frequency of advanced stage disease at clinical diagnosis in the absence of screening and disease-specific survival by stage in the absence of treatments of interest. These datapoints are often unavailable or are available only for certain facilities or areas of the country. We used historical stage distributions from the Connecticut tumour registry as a proxy for stage at diagnosis in the absence of screening. Although this proxy is not ideal, the prevalence of advanced-stage disease at diagnosis from this cohort is similar to that reported in hospital-based retrospective case reviews from the East African region. Even if reliable sources of data are available, the user will have to extract model-relevant inputs from incomplete or inconsistent data sources. Partially screened or treated populations are common and can make modelling difficult. In Colombia, for example, opportunistic mammographic screening is available but it is not universally used, and some of the population have access to systemic therapies. Thus, we modelled expanded access to existing screening or treatment interventions rather than to new ones. Regardless, the model still requires information on baseline survival in the absence of screening or treatment. The appendix includes a worked example of a calculation for this purpose, which is similar to that reported by Welch and Passow41 for a partially screened population.
Model inputs for early detection and treatment can also be challenging to estimate. For early detection, the anticipated shifts in disease stage associated with candidate screening strategies depend on the completeness and quality of screening implementation in the target population, which might have logistical determinants that are dependent on location. Zelle and colleagues42 used a mathematical model to predict stage shift as a result of screening in LMICs, but this model requires specification of other parameters pertaining to disease natural history, which are often unknown. We modelled expected stage shifts using data from the control group (clinical breast examination only) of the Canadian breast screening trial.16–18 Although not ideal, this result is a proxy for the effects of clinical breast examinations in an unscreened population. Our interface allows users to modify this input to better reflect their specific setting. Only two stages are considered because the number of specific downstaging options increases substantially as the number of stages increases, and data on the frequency of each option are not available. For treatment, efficacy estimates from randomised controlled trials represent a highly controlled, generally compliant setting across multiple cycles of therapy; because full compliance with such regimens might not be achievable in some LMICs, estimates can be overly optimistic.
Given the uncertainty that is inherent to model inputs for LMICs, our modelling framework permits assessment of uncertainty related to specified plausible upper and lower bounds for model inputs. Sensitivity analysis can be done via the user interface by substituting given values of the upper and lower bounds for any input parameter.
This first version of our modelling framework has a restricted number of outcomes and does not investigate the effect of modelled interventions on quality-adjusted and disability-adjusted life-years. The addition of these outcomes to the model will be straightforward if the requisite inputs are available. We do not provide data on cost-benefit because cost estimates are often specific to each situation and can change rapidly. In middle-income and high-income countries, overdiagnosis is a major concern and occurs when indolent or slow-growing tumours are identified by sensitive screening methods available in these countries. Overdiagnosis and overtreatment are not a problem in countries where most women present with locally advanced or metastatic disease and screening methods are typically less technologically advanced.
In summary, although the implementation of screening and treatment policies for breast cancer control that have shown success in high-income settings might be beyond the reach of many countries, our model is a tool for pragmatic decision making in LMICs. It allows assessment of the benefits of more modest improvements in awareness and access to diagnosis alone, or in combination with improvements in access to appropriate treatments. Ultimately, our model allows policy makers to focus on strategies that are affordable and practical in their particular setting, thus facilitating the identification of resource-appropriate policies, rather than a one-size-fits-all approach.43
Supplementary Material
Research in context Evidence before the study
We searched PubMed for articles published in English between Jan 1, 2005, and Dec 31, 2017, using the search terms “LMIC” OR “low-income”, “policy”, “prediction”, “decision-making tool”, “population outcomes” NOT “personalized”, “models” AND/OR “simulation”, “breast cancer” AND/OR “screening” AND/OR “treatment”, “breast-cancer specific survival”, and “treatment specific survival”. This search showed no models are accessible to policy makers that permit direct comparisons of the effect of systemic treatment or early diagnosis interventions on breast cancer-specific population-based outcomes, using data specific to low-income and middle-income settings. Many existing models focus on risk analyses for individuals, such as prognostic models for treatment decisions. Models that are focused on policy generally rely on complex natural history assumptions (such as models from the Cancer Intervention and Surveillance Modeling Network consortium that are used to support mammographic screening recommendations in the USA) and most often relate to high-income countries. An exception is the work of Zelle and colleagues; their models project the cost-effectiveness of interventions for breast cancer control in low-income and middle-income countries (LMICs). These models also rely on underlying natural history to project the shift in cancer stage in response to screening; however, the parameters driving the model relate to latent events in disease progression and are not easily estimated in different settings. Our model simplifies this concept by allowing the user to specify the expected stage shift via an accessible user interface that is publicly available.
Added value of this study
Making evidence-based choices that are appropriate for the availability of resources is crucial to successful strategies for breast cancer control. Breast cancer incidence is increasing in many LMICs and scarce economic resources prevent adequate investment in early diagnosis and treatment programmes, resulting in poor outcomes for women. Policy makers often do not have access to evidence-based guidelines that are specific to their resource setting or geographic region. Most trials on breast screening and treatment are done in high-income countries, where health care provision is unfortunately far better than it is in most LMICs. We have previously developed an accessible modelling framework that separates the benefits of breast cancer screening from the benefits of treatment. Here, we describe the application of this framework to support evidence-based policy decisions in LMICs. The framework projects the effects of combinations of breast early diagnosis programmes (eg, education efforts or clinical breast examinations) and treatment options (eg, oestrogen-receptor testing and targeted endocrine treatment) on breast cancer-related mortality. Our online interface allows users to directly model different policies for breast cancer control and observe their population-level effects on breast cancer mortality. Our model has the specific advantage of allowing users to utilise region-specific data for baseline breast cancer incidence and survival and also accommodates partial dissemination of the interventions considered.
Implications of all the available evidence
Our model quantifies the benefits of access to early detection versus access to adjuvant treatments in two settings with different resource constraints. The findings show that different combinations of interventions for early detection and treatment might result in similar reductions in mortality, thus providing an opportunity for policy makers in LMICs to make decisions on the basis of their resources and infrastructure capabilities.
Acknowledgments
This work was supported by the Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Cancer Center Support Grant of the US National Institutes of Health (P30 CA015704). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. BOA was supported by funding from Susan G Komen for the Cure Leadership Grant (SAC110001).
Funding Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Cancer Center Support Grant of the US National Institutes of Health.
Footnotes
Contributors
BOA, RE, and JKB conceived and designed the study. JKB did the programming. All authors reviewed the literature, interpreted the data, and wrote and revised the manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64: 252–71. [DOI] [PubMed] [Google Scholar]
- 2.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353: 1784–92. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008; 9: 730–56. [DOI] [PubMed] [Google Scholar]
- 4.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer 2008; 113: 2221–3. [DOI] [PubMed] [Google Scholar]
- 5.Shyyan R, Sener SF, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: diagnosis resource allocation. Cancer 2008; 113: 2257–68. [DOI] [PubMed] [Google Scholar]
- 6.Eniu A, Carlson RW, El Saghir NS, et al. Guideline implementation for breast healthcare in low- and middle-income countries: treatment resource allocation. Cancer 2008; 113: 2269–81. [DOI] [PubMed] [Google Scholar]
- 7.Carlson RW, Scavone J, Koh W- J, et al. NCCN Framework for resource stratification: a framework for providing and improving global quality oncology care. J Natl Compr Canc Netw 2016; 14: 961–69. [DOI] [PubMed] [Google Scholar]
- 8.Birnbaum J, Gadi VK, Markowitz E, Etzioni R. The effect of treatment advances on the mortality results of breast cancer screening trials: a microsimulation model. Ann Intern Med 2016; 164: 236–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu KC, Smart CR, Tarone RE. Analysis of breast cancer mortality and stage distribution by age for the Health Insurance Plan clinical trial. J Natl Cancer Inst 1988; 80: 1125–32. [DOI] [PubMed] [Google Scholar]
- 10.Feuer EJ, Mariotto A, Merrill R. Modeling the impact of the decline in distant stage disease on prostate carcinoma mortality rates. Cancer 2002; 95: 870–80. [DOI] [PubMed] [Google Scholar]
- 11.Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies: model estimates of potential benefits and harms.Ann Intern Med 2013; 158: 145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN guidelines insights: breast cancer, version 1.2017 J Natl Compr Canc Netw 2017; 15: 433–51. [DOI] [PubMed] [Google Scholar]
- 13.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378: 771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012; 379: 432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutler SJ, Ederer F, Griswold MH, Greenberg RA. Survival of breast-cancer patients in Connecticut, 1935–54. J Natl Cancer Inst 1959; 23: 1137–56. [PubMed] [Google Scholar]
- 16.Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40 to 49 years. CMAJ 1992; 147: 1459–76. [PMC free article] [PubMed] [Google Scholar]
- 17.Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ 1992; 147: 1477–88. [PMC free article] [PubMed] [Google Scholar]
- 18.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ 2014; 348: g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Autier P, Hery C, Haukka J, Boniol M, Byrnes G. Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening. J Clin Oncol 2009; 27: 5919–23. [DOI] [PubMed] [Google Scholar]
- 20.Jedy-Agba E, McCormack V, Adebamowo C, dos-Santos-Silva I.Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2016; 4: e923–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenzie F, Zietsman A, Galukande M, et al. Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer—disparities in outcomes (ABC-DO) study. Int J Cancer 2018; 142: 1568–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murillo R, Diaz S, Perry F, et al. Increased breast cancer screening and downstaging in Colombian women: a randomized trial of opportunistic breast-screening. Int J Cancer 2016; 138: 705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murillo R, Diaz S, Sanchez O, et al. Pilot implementation of breast cancer early detection programs in Colombia. Breast Care 2008; 3: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Agency for Research on Cancer. CI5 I-X: cancer incidence in five continents. 2016. http://ci5.iarc.fr/CI5I-X/Pages/download.aspx (accessed June 28, 2018).
- 25.Pineros M, Sanchez S, Cendales R, Perry F, Garcia RO. Caracteristicas sociodemograficas, clinicas y de la atencion de mujeres con cancer de mama en Bogota. Rev Colomb Cancerol 2008; 12: 181–90. [Google Scholar]
- 26.Eng A, McCormack V, dos-Santos-Silva I. Receptor-defined subtypes of breast cancer in indigenous populations in Africa: a systematic review and meta-analysis. PLoS Med 2014; 11: e1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serrano-Gomez SJ, Sanabria-Salas MC, Hernandez-Suarez G, et al. High prevalence of luminal B breast cancer intrinsic subtype in Colombian women. Carcinogenesis 2016; 37: 669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galukande M, Wabinga H, Mirembe F. Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: a cohort study. World J Surg Oncol 2015; 13: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantelhardt EJ, Zerche P, Mathewos A, et al. Breast cancer survival in Ethiopia: a cohort study of 1,070 women. Int J Cancer 2014; 135: 702–09. [DOI] [PubMed] [Google Scholar]
- 30.Bravo LE, Garcia LS, Carrascal E, Rubiano J. Burden of breast cancer in Cali, Colombia: 1962–2012. Salud Publica Mex 2014; 56: 448–56. 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet 2005; 366: 572–78. [DOI] [PubMed] [Google Scholar]
- 32.Cronin KA, Mariotto AB, Clarke LD, Feuer EJ. Additional common inputs for analyzing impact of adjuvant therapy and mammography on U.S. mortality. J Natl Cancer Inst Monogr 2006; 36: 26–29. [DOI] [PubMed] [Google Scholar]
- 33.Ilbawi AM, Anderson BO. Cancer in global health: how do prevention and early detection strategies relate? Science Transl Med 2015; 7: 278cm1. [DOI] [PubMed] [Google Scholar]
- 34.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide. 2012. http://globocan.iarc.fr/Pages/burden_sel.aspx (accessed June 28, 2018).
- 35.Mandelblatt J, Schechter CB, Lawrence W, Yi B, Cullen J. The SPECTRUM population model of the impact of screening and treatment on U.S. breast cancer trends from 1975 to 2000: principles and practice of the model methods. J Natl Cancer Inst Monogr 2006; 36: 47–55. [DOI] [PubMed] [Google Scholar]
- 36.Fryback DG, Stout NK, Rosenberg MA, Trentham-Dietz A, Kuruchittham V, Remington PL. The Wisconsin breast cancer epidemiology simulation model. J Natl Cancer Inst Monogr 2006; 36: 37–7 [DOI] [PubMed] [Google Scholar]
- 37.Plevritis SK, Salzman P, Sigal BM, Glynn PW. A natural history model of stage progression applied to breast cancer. Stat Med 2007; 26: 581–95. [DOI] [PubMed] [Google Scholar]
- 38.Tan SY, van Oortmarssen GJ, de Koning HJ, Boer R, Habbema JD. The MISCAN-Fadia continuous tumor growth model for breast cancer. J Natl Cancer Inst Monogr 2006; 36: 56–65. [DOI] [PubMed] [Google Scholar]
- 39.Zelle SG, Nyarko KM, Bosu WK, et al. Costs, effects and cost-effectiveness of breast cancer control in Ghana. Trop Med Int Health 2012; 17: 1031–43. [DOI] [PubMed] [Google Scholar]
- 40.Zelle SG, Vidaurre T, Abugattas JE, et al. Cost-effectiveness analysis of breast cancer control interventions in Peru. PLoS One 2013; 8: e82575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014; 174: 448–54. [DOI] [PubMed] [Google Scholar]
- 42.Zelle SG, Baltussen R, Otten JD, Heijnsdijk EA, van Schoor G, Broeders MJ. Predicting the stage shift as a result of breast cancer screening in low-and middle-income countries: a proof of concept. J Med Screen 2015; 22: 8–19. [DOI] [PubMed] [Google Scholar]
- 43.Harford JB. Breast-cancer early detection in low-income and middle-income countries: do what you can versus one size fits all. Lancet Oncol 2011; 12: 306–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


