Abstract
Enriched environment treatment (EET) is a potential intervention for depression by inducing brain-derived neurotrophic factor (BDNF). However, its age dependency remains unclear. We recently found that EET during early-life development (ED) was effective in increasing exploratory activity and anti-despair behavior, particularly in promoter IV-driven BDNF deficient mice (KIV), with the largest BDNF protein induction in the hippocampus and frontal cortex. Here, we further determined age dependency of EET effects on anhedonia and promoter-specific BDNF transcription, by using the sucrose preference test and qRT-PCR. Wild-type (WT) and KIV mice received two months of EET during ED, young-adulthood and old-adulthood (0–2, 2–4, and 12–14 months, respectively). All KIV groups showed reduced sucrose preference, which EET equally reversed regardless of age. EET increased hippocampal BDNF mRNA levels for all ages and genotypes, but increased frontal cortex BDNF mRNA levels only in ED KIV and old WT mice. Transcription by promoters I and IV was age-dependent in the hippocampus of WT mice: more effective induction of exon IV or I during ED or old-adulthood, respectively. Transcription by almost all 9 promoters was age-specific in the frontal cortex, mostly observed in ED KIV mice. After discontinuance of EET, the EET effects on anti-anhedonia and BDNF transcription in both regions persisted only in ED KIV mice. These results suggested that EET was equally effective in reversing anhedonia and inducing hippocampal BDNF transcription, but was more effective during ED in inducing frontal cortex BDNF transcription and for lasting anti-anhedonic and BDNF effects particularly in promoter IV-BDNF deficiency.
Keywords: enriched environment, across ages, anhedonia, brain-derived neurotrophic factor (BDNF), gene expression, promoter IV, early life
Introduction
Enriched environment treatment (EET), which combines physical exercise, mental stimulation, and social interaction (Hebb, 1947, Rosenzweig, 1966, Segovia et al., 2009, Van Praag et al., 2000), is a potential intervention for depression (Dimeo et al., 2001, Greenwood et al., 2003, Martinsen, 1990). EET induces expression of brain-derived neurotrophic factor (BDNF) (Bennett et al., 1969, Greenough & Volkmar, 1973, Jha et al., 2011, Kempermann et al., 1997, Molteni et al., 2002, Van Praag et al., 1999), a critical neuronal growth factor implicated in the pathophysiology of depression (Boulle et al., 2012, Castren, 2005, Chourbaji et al., 2011, Duman & Monteggia, 2006, Sakata, 2011, Sakata, 2014). Decreased BDNF expression has been observed in the hippocampus and prefrontal cortex of depressed humans (Dwivedi et al., 2003) and stressed animals (Roth et al., 2009, Smith et al., 1995, Tsankova et al., 2006). In particular, inactivation of promoter IV via epigenetic modification is linked to depression (Hing et al., 2012, Keller et al., 2010) and stress (Fuchikami et al., 2009, Roth et al., 2011, Tsankova et al., 2006). Promoter IV-driven BDNF deficiency leads to depression-like behavior in mice (Sakata et al., 2010). We previously found that EET was more effective than treatment with four classes of antidepressant drugs in reversing the depression-like behavior caused by the promoter IV-BDNF deficiency in young-adult mice (Jha et al., 2011, Sakata et al., 2013b). While 9 promoters control BDNF gene expression in both humans and rodents (Aid et al., 2007, Liu et al., 2005), EET induces BDNF expression driven by promoters I, II, and III (Adlard et al., 2005, Jha et al., 2011, Russo-Neustadt et al., 2000, Zajac et al., 2010) with epigenetic modification at promoters II, IV, and VI (Gomez-Pinilla et al., 2010, Kuzumaki et al., 2011).
Despite the well-established BDNF effects of EET, what remains unclear is its age dependency, particularly for effectiveness against depression. Does EET affect depression differently across ages? This information is important in developing effective strategies for prevention and treatment of depression. We recently examined EET effects across three distinctive life stages in mice: early-life development, young-adulthood, and old-adulthood. We found that EET effects in increasing exploratory activity and anti-despair behavior were largest and long-lasting when EET was provided during early-life development, particularly in promoter IV-BDNF deficient mice (Jha et al., 2016). BDNF protein induction in the hippocampus and frontal cortex was also the largest after early-life EET, while the EET effects were limited in old-adult mice (Jha et al., 2016). These results led to our hypothesis that EET during early-life development provides maximum and lasting effects in antidepressive behavior and BDNF induction. In continuation of this study, we further tested our hypothesis by investigating age dependency of EET effects on anhedonia, one core depression-related phenotype. We also determined long-lasting transcriptional changes driven by each BDNF promoter, which may account for lasting BDNF protein induction after discontinuance of EET.
Materials and Methods
Animals
Wild-type (WT) and knock-in BDNF-promoter IV (KIV) mice were used to assess EET effects in normal and depressed conditions (Sakata et al., 2010), respectively. The generation of KIV mice has been described previously (Sakata et al., 2009) and followed the breeding guidelines recommended (Crusio et al., 2009). Briefly, KIV mice lack promoter IV-driven BDNF expression by insertion of a green fluorescent protein (GFP) gene, but retain 8 other promoters and the BDNF protein-coding region (Sakata et al., 2009). KIV mice were generated from 129cX/Sv × 129X1/SvJ ES cells and then crossed to C57BL/6J females for more than 12 generations. Heterozygous mice were bred to produce WT and KIV littermates of the same genetic background. Offspring from these littermates were used.
EET effects were examined at three life stages: early-life development (ED: 0–2 months), young-adult (YA: 2–4 months), and old-adult (OA: 12–14 months); these life stages represent development before reproductive maturity, after sexual maturity, and middle-age in mice (Flurkely et al., 2007, Jax). Male and female mice were used to examine sex-specific effects.
A cohort of mice was used to assess EET effects on anhedonia (about 360 mice: N=15 mice per group × 2 genotypes × 2 sexes × 2 treatment conditions × 3 life stages). Another cohort of mice was used to assess EET effects on BDNF gene transcription (about 192 mice: N=8 mice of 4 males and 4 females per group × 2 genotypes × 2 treatment conditions × 2 time points × 3 life stages). The sample size was based upon previous experiments and power analyses. The same animals in our previous study (Jha et al., 2016) were used in this study. Mice were group-housed with standard bedding in a climate-controlled vivarium on a normal 12-hour dark/light cycle with ad libitum access to food and water. All animal experiments were approved by the University of Tennessee Laboratory Animal Care and Use Committee.
Treatments
Age- and sex- matched mice were randomly assigned to standard condition treatment (SCT) or enriched environment treatment (EET), as described previously (Jha et al., 2011, Jha et al., 2016). Briefly, SCT consisted of a small 27×16×12 cm cage containing 2–5 mice, group-housed to avoid isolation stress. EET consisted of a larger 44×22×16 cm cage, containing one plastic running wheel per 5 mice to allow for physical activity, a variety of toys to increase perception and mental exercise, and 5–10 company mice with nesting material to increase social interaction. Toys were replaced weekly, and mice in EET cages were given bacon-flavored Rodent Foraging Crumbles (Bio-Serv, Frenchtown, NJ) to encourage exploration in the enriched environment.
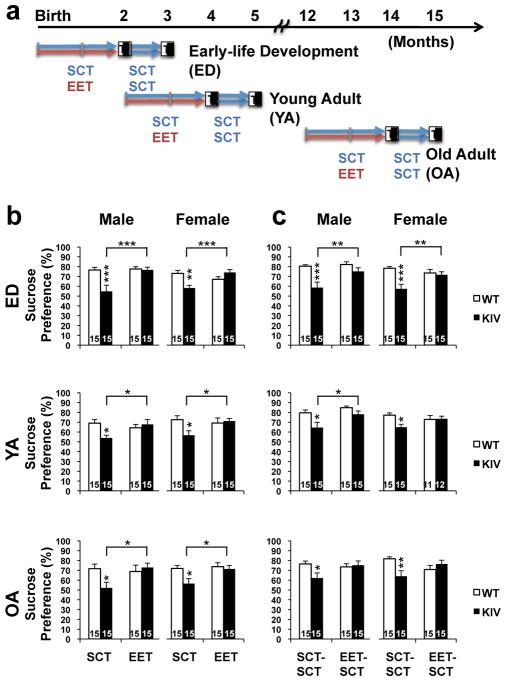
The treatment period of two-months was used to encompass mouse development from birth to reproductive maturity. ED-EET mice were born and raised in an EET cage, then weaned to another EET cage at 3 weeks of age until 2 months of age. YA-EET and OA-EET mice started treatment at 2 months or 12 months of age, respectively. After treatment, EET mice were then placed in standard condition (EET-SCT), while SCT mice remained in standard condition (SCT-SCT), for 1 additional month to determine, if any, the persisting effects of EET (see Fig. 1a for research design).
Fig. 1.
a. Research design WT and KIV mice received 2 months of enriched environment treatment (EET; red arrows) or standard condition treatment (SCT: blue arrows) from birth (early-life development: ED), 2 months of age (young-adult: YA), or 12 months of age (old-adult: OA), then received 1 month of SCT. Anhedonia-like behavior and BDNF transcript levels were measured after 2 months of EET (test 1: T1) and consequent 1 month of SCT (test 2: T2). Reused from Jha et al. Transl. Psychiatry 2016. b and c. Effects of BDNF deficiency and EET on sucrose preference across ages tested after EET (b, SCT/EET, at T1), then after 1 month of SCT (c, SCT-SCT/EET-SCT, at T2). Asterisks on the bars show a significant difference between genotypes. Note that all KIV groups showed reduced sucrose preference and that EET reversed it regardless of age and sex, which lasted 1 month after discontinuance with significant increases in KIV ED males and females and YA males. N=11–15 per group (shown at the bar). *P<0.05, **P<0.01, ***P<0.005.
Sucrose Preference Test
This test was used to assess mouse anhedonia (Pothion et al., 2004, Sakata et al., 2010) after the initial two months of SCT or EET and, again, after one month of SCT (at T1 and T2 in Fig. 1a). Mice were habituated to two water bottles for 4 days and then water-deprived overnight (17:00–9:00 h). During a 3 h session (9:00–12:00 h), mice were housed individually and given access to one bottle of regular water and one bottle of preferable 1% sucrose solution. Liquid consumption was measured by weighing each bottle before and after consumption, and sucrose preference was calculated as total sucrose water intake/total (sucrose + water) intake (Willner et al., 1987).
RNA Extraction and qRT-PCR by BioMark
qRT-PCR was used to measure total BDNF mRNA levels and to identify the involved promoters by detecting each transcript driven by 9 BDNF promoters. Hippocampus and frontal cortex tissues were collected between 14:00–17:00 h from the 4 treatment groups (SCT/EET at T1 and SCT-SCT/EET-SCT at T2, see Fig. 1a) and stored at −80°C until processed. Each sample was homogenized by pipet and QIAshredder (Qiagen, Valencia, CA). Total RNA was extracted using an RNeasy Kit with on-column DNase (Qiagen) and quantified using the NanoDrop spectrophotometer (Agilent Technologies, Santa Clara, CA). One μg of total RNA was converted to cDNA (First Strand cDNA Synthesis Kit, Roche Applied Science, Indianapolis, IN). qRT-PCR was performed using BioMark, as described previously (Reiner et al., 2012, Sakata & Duke, 2014, Sakata & Overacre, 2017). Briefly, 25 ng of cDNA was preamplified with pooled primers (200 nM) and PreAmp Master Mix (Applied Biosystems, Carlsbad, CA) for 14 cycles of 95°C for 15 s and 60°C for 4 min. Then diluted reactions (1:5) with TE buffer (1 mM Tris-HCl pH 8 and 0.1 mM EDTA) were used for qPCR in a M96.96 dynamic array chip (Fluidigm, South San Francisco, CA). Each inlet contained 5 μL of assay mix [1 μM of forward primer, 1 μM of reverse primer, 1 μM of UPL probe, and 1× assay reagent (Fluidigm, PN85000736)] or 5 μL of sample mix [2.25 μL of preamplified sample, 2.5 μL of 2× Kapa Probe Fast qPCR Master Mix (Kapa Biosystems, Wilmington, MA) and 0.25 μL of 20× Sample Loading Solution (Fluidigm, PN85000735)]. The thermal cycle consisted of 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Cycle threshold (Ct) values were obtained by BioMark Gene Expression Data Analysis software after automatic inspection for quality. The average Ct values of cyclophilin D and HGPRT were used for reference because these genes previously showed the least variation of expression in the hippocampus and frontal cortex among six tested housekeeping genes (cyclophilin D, HGPRT, TBP, β-actin, β-tubulin, and S19). Relative gene expression values were determined by using the 2−ΔΔCt method of Livak and Schmittgen (Livak & Schmittgen, 2001). Gene expression values were normalized to the average values of gene expression in control mice (e.g., respective SCT groups). Information on primers and probes is presented in Supplementary Table 1.
Statistical Analysis
The D’Agostino-Pearson omnibus normality tests or Shapiro-Wilk tests were performed to analyze the distribution of data for sucrose preference or gene expression, respectively, in each genotype and each treatment condition. All the data were normally distributed (P>0.05). Two-way analyses of variance (ANOVA) with post hoc Bonferroni multiple comparisons were computed to determine two effects (e.g., effects of EET and age) unless specified. One-way ANOVA was used on three data groups (ED, YA, vs. OA). Student’s t-tests were performed on the two specific data groups (e.g., KIV-EET vs. WT-SCT). Data are presented as means ± SEM with statistical significance set at P<0.05*, P<0.01**, and P<0.005***.
Results
1. Genetic and environmental effects on anhedonia
1-1. Genotype effects
We first determined whether promoter IV-BDNF deficiency affected sucrose preference differently across life stages. When compared to WT mice in SCT, all KIV mice in SCT showed significantly reduced sucrose preference (WT vs. KIV: P<0.05 for each, detailed statistics are presented in Supplementary Table 2, Fig. 1b). No age effects were observed in the reduced sucrose preference of KIV mice of either sex (ED, YA, and OA: Fmale (2,28)=0.3, P>0.05, Ffemale (2,28)=0.4, P>0.05, one-way ANOVA, Supplemental Fig. 1a), indicating that promoter IV-BDNF deficiency universally caused anhedonia.
1-2. EET effects
Next, we asked whether EET effects on anhedonia are age-dependent. EET across all ages significantly increased sucrose preference of KIV mice for both sexes (KIV, SCT vs. EET: P<0.05 for each, see Supplementary Table 2 for statistical details), while normalizing its reduction to the levels of WT mice in SCT (WT-SCT vs. KIV-EET: P>0.05, Student-t test, Fig. 1b). No age-specific effects were observed in the EET effects for either sex (Fmale(2,84)=2.1, P>0.05, Ffemale(2,83)=0.04, P>0.05, Supplementary Fig. 1b). EET did not change the sucrose preference in WT mice at any life stage (WT, SCT vs. EET: P>0.05 at any ages, Fig. 1b). These results indicated that EET reversed anhedonia caused by BDNF deficiency, regardless of age and sex, but did not increase sucrose preference in normal conditions.
1-3. Lasting EET effects
We then investigated whether the EET effects in reversing anhedonia would persist without EET and whether the effects differ across ages. One month after EET discontinuance, all KIV groups with EET presented similar sucrose preference levels as WT mice (KIV EET-SCT vs. WT SCT-SCT: P>0.05 at any ages). Significant EET-induced increases were observed only in ED KIV males and females and YA KIV males (KIV SCT-SCT vs. EET-SCT: P<0.05 for each, Fig. 1c). These results indicated that EET effects in reversing anhedonia persisted, regardless of age and sex, with significant effects when EET was provided in early life.
2. Total BDNF mRNA levels across ages
We next examined the age-dependent effects of promoter IV-BDNF deficiency and EET on BDNF gene transcription. Total BDNF mRNA levels were measured by the levels of exon IXc, the common BDNF protein-coding region shared by transcripts driven by all 9 BDNF promoters (Aid et al., 2007, Liu et al., 2005).
2-1. Basal levels
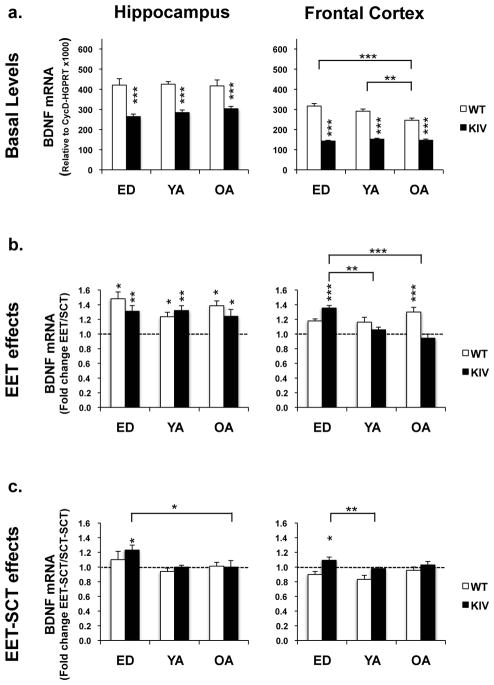
Age effects on basal BDNF levels without EET were examined by comparing SCT groups at ED, YA, and OA. Only WT frontal cortex showed age-dependent decline of basal BDNF levels (WT-SCT, OA vs. ED or YA: P<0.05, Fig. 2a). KIV mice presented significant reductions in total BDNF mRNA levels in both frontal cortex and hippocampus, regardless of age, when compared to WT mice (WT-SCT vs. KIV-SCT: P<0.001 for each, Fig. 2a).
Fig. 2.
Total BDNF mRNA levels (the protein-coding exon IXc) in the hippocampus (left) and frontal cortex (right) at early-life development (ED), young-adult (YA), and old-adult (OA). a. Basal levels in standard conditions. Asterisks on the columns show significant differences between genotypes. N=14–16 per group. b. EET effects shown by fold changes of BDNF levels in mice with EET divided by those with SCT. Asterisks on the columns show significant BDNF induction in EET group compared to SCT group. N=6–8 per group. c. Lasting effects of EET shown by fold changes of BDNF levels in mice with EET and consequent SCT (EET-SCT) divided by levels in SCT mice (SCT-SCT). N=6–8 per group. *P<0.05; **P<0.01; ***P<0.005.
2-2. EET effects
In the hippocampus, EET significantly increased total BDNF mRNA levels in all age groups for both genotypes (SCT vs. EET: at least P<0.05, Fig. 2b left). No significant effects of aging and genotype were observed (P>0.05, Supplementary Table 2, 3). By contrast, in the frontal cortex, significant effects of age and genotype were observed (Fage(2,42)=4.4, P<0.05; Fgenotype(1,42)=9.2, P<0.01), where EET increased total BDNF mRNA levels only in ED KIV mice and OA WT mice (SCT vs. EET: P<0.005 for each, Fig. 2b right).
2-3. Lasting EET effects
One month after EET discontinuance, treatment × age interactions were observed for both regions (Fhippocampus(2,38)=6.28, P<0.01; Ffrontal_cortex(1,42)=3.6, P<0.05) where only ED KIV mice showed significant BDNF increases (KIV, EET-SCT vs. SCT-SCT: P<0.05, Fig. 2c). The results indicated that BDNF gene induction persisted only with early-life EET in promoter IV-BDNF deficiency, possibly due to lasting compensation of other BDNF promoter activity for the promoter IV defect.
3. EET activation of BDNF promoters across ages
We further examined which promoters contributed to EET-induced BDNF transcription. We examined activity of 9 promoters by measuring mRNA levels of the immediately downstream BDNF exons I-IXa (Aid et al., 2007, Liu et al., 2005).
3-1. Basal levels of each BDNF exon
WT mice showed age-dependent decline of all BDNF exons except for exon V in the hippocampus, (ED vs. OA: at least P<0.05 for each, Supplementary Fig. 2 left). WT mice showed age-dependent decline of only exon I in the frontal cortex (OA vs. ED: P<0.005, Supplementary Fig. 2 right), which likely accounted for the age-dependent decline of total BDNF mRNA levels (exon IXc, Fig. 2 right). KIV mice showed age-dependent decline of only exons I in both hippocampus and frontal cortex (YA vs. OA: at least P<0.05, Supplementary Fig. 2). KIV mice, when compared to WT mice, showed reduced levels of almost all exons in both regions in all age groups (except VI in YA groups) (WT vs KIV: P<0.05, Supplementary Fig. 2), which reproduced previous findings in YA KIV mice (Martinowich et al., 2011, Maynard et al., 2016). Promoter IV-BDNF deficiency may reduce other promoter activity, regardless of age.
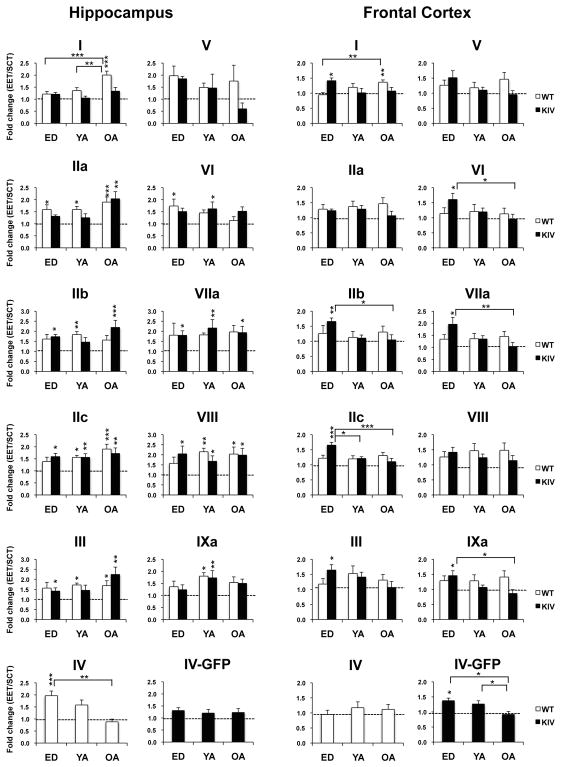
3-2. EET effects across ages
In the hippocampus, both WT and KIV mice showed significant EET effects for almost all exons (at least P<0.05, except for exon V in KIV mice, Supplementary Table 2). EET significantly increased levels of exons IIa, IIb, IIc, III, VI, VIIa, VIII, and IXa (EET vs. SCT: at least P<0.05) without any age or genotype effects (among ED, YA and OA or WT vs. KIV: P>0.05, Fig. 3 left), which indicated universal EET activation of the respective promoters. By contrast, EET effects on exons I and IV were age- and genotype-specific; EET increased levels of exon I only in OA WT mice and exon IV only in ED WT mice (EET vs. SCT: P<0.005 for each, Fig. 3 left) with significant genotype differences (WT-EET vs. KIV-EET: at least P<0.01). The results indicated that EET activated promoter IV more effectively during early-life development than adulthood, but activated promoter I more effectively in aged stages in the hippocampus of normal mice.
Fig. 3. EET effects on BDNF transcription by 9 promoters across ages.
The data show fold changes of exon levels in mice with EET divided by those in mice with SCT in the hippocampus (left) and frontal cortex (right). Asterisks on the columns show significant changes in levels between EET groups vs. SCT groups. ED, early-life development; YA: young-adult; OA: old-adult. N=6–8 per group. *P<0.05; **P<0.01; ***P<0.005.
In the frontal cortex, EET significantly increased levels of almost all exons (I, IIb, IIc, III, IV, VI, VIIa, and IXa) only in ED KIV mice (SCT vs. EET: P<0.05), where significant age effects were observed (KIV, ED vs. OA: P<0.05, Fig. 3 right). One exception was that EET increased levels of exons I in the OA WT group (SCT vs. EET: P<0.05) with a significant age effect (WT, ED vs. OA: P<0.01, Fig. 3 right). These results indicated that EET activated various BDNF promoters in the frontal cortex more effectively when provided during ED than in adulthood, only in promoter IV-BDNF deficient condition, while EET may compensate for age-dependent decline in total BDNF mRNA levels in normal aging by activating promoter I.
3-3. Lasting BDNF promoter activation by EET
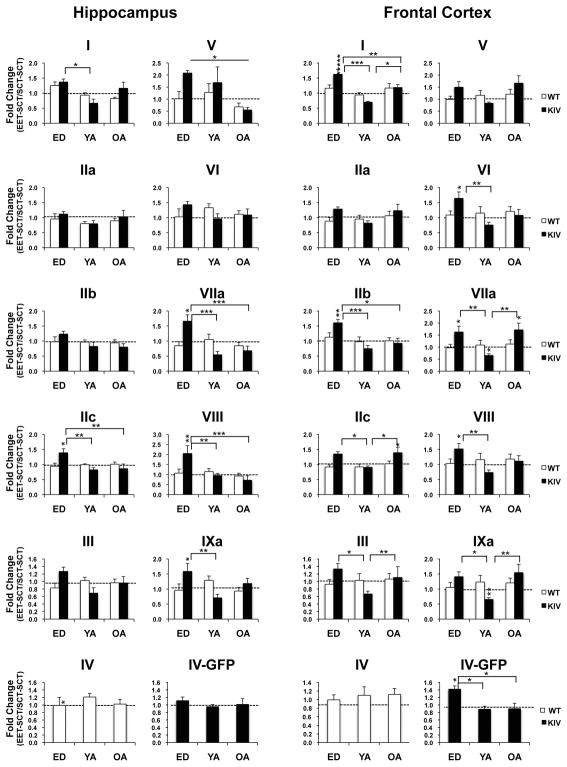
Interestingly, after one month discontinuance of EET, only ED KIV mice showed significant increases of promoter-specific transcriptions of BDNF, exons IIc, VIIa, VIII, IXa in the hippocampus and exons I, IIb, VIIa, and VIII in the frontal cortex (EET-SCT vs. SCT-SCT: P<0.05), where significant age were observed (ED vs. YA/OA: at least P<0.05, Fig. 4). These results indicated sustained activity of several promoters after EET, only when EET was provided during early life, specifically in the promoter IV-deficient condition.
Fig. 4. Lasting effects of EET on promoter-specific BDNF transcription across ages.
The data show fold changes of exon levels in mice with EET and subsequent SCT divided by those with SCT-SCT in the hippocampus (left) and frontal cortex (right). Asterisks on the columns show significant changes in levels between EET-SCT vs. SCT-SCT groups. Note the sustained induction of some exons after EET discontinuance particularly in ED KIV mice in both regions. N=6–8 per group.
*P<0.05; **P<0.01; ***P<0.005.
Discussion
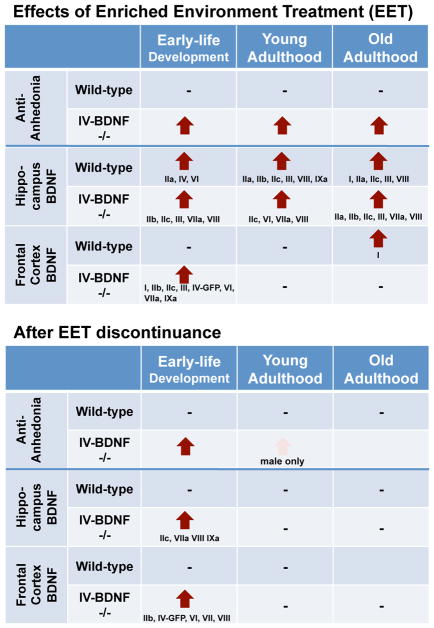
Results of the present study demonstrated four major findings: 1) BDNF deficiency commonly led to anhedonia, normalized by EET, regardless of age and sex; 2) EET increased BDNF mRNA levels regardless of age and genotypes in the hippocampus but only during KIV ED and WT OA in the frontal cortex; 3) almost all BDNF promoters (I-IX) contributed to BDNF gene induction by EET for both brain regions, where age- and genotype-dependency was observed for promoters I and IV in the hippocampus and almost all promoters in the frontal cortex; and 4) the EET effects in increasing sucrose preference and promoter-specific BDNF transcription persisted only in KIV mice when EET was provided during early life (Fig. 5). Altogether, these findings partially supported our hypotheses: EET was equally effective in reversing anhedonia and inducing hippocampal BDNF levels, regardless of age, but was more effective during early life in inducing frontal cortex BDNF levels and for lasting anti-anhedonic and BDNF effects, particularly in promoter IV-BDNF deficiency. To our knowledge, this is the first study that showed effects of BDNF deficiency and EET on anhedonia and promoter-specific BDNF transcription across ages; many other studies, including ours, have shown these effects mostly in one age stage.
Fig. 5. Effects of EET on anhedonia and BDNF transcription across ages.
The arrows show significant increases in sucrose preference and total BDNF mRNA levels. Promoter-specific BDNF transcripts are shown in Roman numbers.
1. Anhedonia
Previous studies have shown that BDNF deficiency reduces sucrose preference in young-adult KIV males (Jha et al., 2011, Sakata et al., 2010) and CaMKII promoter-controlled BDNF knockout females (Monteggia et al., 2007). This study expanded these findings across ages for both males and females. KIV mice also show reduced exploratory activity and increased stress-induced despair at any age and sex (Jha et al., 2016). Together, our results suggest that promoter IV-BDNF deficiency commonly causes depression-like behavior, regardless of age and sex.
Our findings of no sex difference in KIV (promoter IV-knockin) mice contrast with sex difference in depression-related behavior in BDNF-coding-region knockout mice (+/− and conditional) (Chourbaji et al., 2008, Monteggia et al., 2007). These knockouts retain promoter IV-driven BDNF expression: half in BDNF+/− mice and intact in regions/cells unaffected by the exogenous promoters (e.g., CaMKII). The BDNF loss in the knockouts may be insufficient to cause anhedonia, but is prone to do so when promoter IV-driven BDNF levels are further reduced under stress (Roth et al., 2009, Tsankova et al., 2006) and females may be more susceptible to stress (Autry et al., 2009). BDNF deficiency in the endogenous-promoter IV-controlled regions/cells (e.g., hippocampal CA1 and prefrontal cortex pyramidal cells) is likely critical to cause depression-related behavior [detailed discussion in (Sakata et al., 2010)].
In humans, depression affects anyone, although the prevalence of depression is sex- and age-dependent: depression is more common among females (5.1%) than males (3.6%), particularly around 60 years of age and in puberty-related teenage children (Who, 2017). Chronic mild stress has been shown to decrease sucrose preference in adult, but not juvenile, rats (Toth et al., 2008). It is possible that the susceptibility to chronic stress, which decreases BDNF expression, may be age- and sex-dependent, whereas BDNF deficiency commonly causes depressive behavior. No age-related changes in sucrose preference were observed in normal WT mice in this study, similar to a previous finding by Tordoff et al. (2007), while age-dependent declines have been reported in >17 months-old rodents (Inui-Yamamoto et al., 2017, Malatynska et al., 2012).
In this study, EET reversed reduced sucrose preference caused by promoter IV-BDNF deficiency, regardless of age. This universal effect of EET in normalizing anhedonia was in contrast to our previous findings of an age-dependent effect of EET in increasing exploratory activity and decreasing stress-induced despair (Jha et al., 2016). These findings together suggest that age dependency of EET efficacy differs among the types of depressive behavior.
Our results showed no sex difference in the EET effects on sucrose preference at any life stage. These results were similar to no sex difference in EET effects on exploratory activity and stress-induced despair (Chourbaji et al., 2008, Jha et al., 2016). These findings together suggest that EET is equally effective in both sexes for reducing depressive behavior caused by BDNF deficiency.
Interestingly, the significant increases in sucrose preference persisted only with early-life EET, similar to the lasting increases in exploratory activity and stress resilience with early-life EET (Jha et al., 2016). These findings together suggest that EET should be started in early life for prevention of depressive behavior later in life.
2. BDNF gene induction by EET across ages
Previously, we had shown that EET-induced levels of BDNF protein were larger in ED than in adult groups (Jha et al., 2016). We hypothesized that this age dependency might be due to more flexible transcriptional regulations earlier in life, which can last longer such as by epigenetic changes. Thus, this study further clarified EET effects on BDNF gene transcription across ages. Our results demonstrated that EET induction of total BDNF mRNA was observed regardless of ages and genotypes in the hippocampus, but only in ED KIV mice and OA WT mice in the frontal cortex. These results suggest that the age dependency of EET effects in increasing BDNF transcription differs among the brain regions. Early life is likely critical for EET to induce BDNF transcription in the frontal cortex particularly in promoter IV-BDNF deficiency. BDNF induction in the OA WT frontal cortex was likely due to the relatively reduced basal levels of BDNF in OA WT mice, suggesting a beneficial effect of EET on normal aging.
Our current results regarding BDNF mRNA levels contained a few discrepancies when compared to our previous results of BDNF protein levels (Jha et al., 2016). First, EET induction of BDNF protein in the hippocampus was more prominent at early life than at old-adulthood (Jha et al., 2016), but that of BDNF mRNA was relatively similar across ages in this study. One possibility is that EET also increases translation of the BDNF transcripts and/or decreases degradation of BDNF protein, and that the post-transcriptional effects in the hippocampus may be larger during early-life development than at old-adulthood. Second, basal levels of BDNF mRNA declined age-dependently in the frontal cortex of WT mice, but those of BDNF protein were similar across ages in our previous study (Jha et al., 2016). The basal levels of BDNF protein may be protected by homeostatic post-translational regulation, such as reduced degradation of BDNF protein at aged stages.
3. Anhedonia related to hippocampal BDNF levels
EET effects in reversing reduced sucrose preference and inducing hippocampal BDNF mRNA were similarly observed across age, whereas EET effects in inducing frontal cortex BDNF mRNA were limited only at ED in KIV mice. These results suggest that BDNF induction by EET in the hippocampus, rather than the frontal cortex, is critical for reducing anhedonia. Taliaz et al. (2010) have shown evidence of hippocampal BDNF directly controlling anhedonia: hippocampus-specific BDNF knockdown reduced sucrose preference while its over-expression normalized a reduction of sucrose preference caused by chronic mild stress in rats. Our result showed increases in hippocampal BDNF but no change in sucrose preference in WT mice, which was also consistent with their findings in normal adult rats (Taliaz et al., 2011).
4. BDNF promoter activity
This study demonstrated that almost all promoters (I-IX), rather than a limited number of promoters, contributed to the BDNF transcription induced by EET in both hippocampus and frontal cortex. These results correspond with the previous studies showing that EET drives multiple BDNF promoters in the hippocampus of YA animals (I, II, III, IV and V) (Adlard et al., 2004, Jha et al., 2011, Russo-Neustadt et al., 2000, Zajac et al., 2010), and expanded similar findings in earlier (ED) and later (OA) in life and in the frontal cortex. Our novel findings were the age-dependent responses of promoters I and IV to EET in the hippocampus and almost all promoters in the frontal cortex. It should be noted that promoters I and IV are activity-dependent, i.e., activated by calcium influx in depolarized neurons (Shieh et al., 1998, Tabuchi et al., 2000, Tao et al., 1998). While our present study measured static levels of BDNF transcripts without neuronal activity (e.g., by learning), EET may also affect neuronal activity-induced BDNF transcription driven by these promoters at specific life stages.
The present study showed that exon I was more effectively induced by EET in OA groups rather than in ED groups in both hippocampus and frontal cortex of WT mice. This result is in accordance with the recent finding by Neidl et al. (2016) that EET in 18–24 month old normal rats significantly increases BDNF transcription by promoter I but not by promoter IV or VI. Promoter I activation by EET involves increases in histone 3 acetylation on a proximal nuclear factor κB site in the promoter region (Neidl et al., 2016). Promoter I can also be activated by inhibiting histone 3 trimethylation (H3K9me) (Snigdha et al., 2016). The current study showed no promoter I activation (exon I) by EET in old-adult KIV mice. This result suggests that the system of nuclear factor κB site acetylation or demethylation at H3K9 to activate promoter I may be defective or interacted by promoter IV-BDNF deficiency.
The results of age-dependency on EET activation of specific BDNF promoters also suggest that EET may affect different types of behavior at different ages because of subregion-specific controls of BDNF promoters. Promoter I is more active in the dentate gyrus, while promoter IV is more active in the CA1 region of the hippocampus (Metsis et al., 1993, Sakata, 2011, Zafra et al., 1990). Therefore, EET activation of promoter I at OA or promoter IV at ED may increase BDNF levels in the dentate gyrus or the CA1, respectively, which can lead to different types of neural effects (e.g., neurogenesis, network activity) and behavior (e.g., pattern separation, learning and memory) (Buzsáki, 2006, Deng et al., 2010, Kempermann et al., 1997, Sakata et al., 2013a, Santarelli et al., 2003).
In the frontal cortex, EET-induced activity of almost all promoters was age-dependent, observed mostly in ED KIV mice. These results agree with the largest induction of total BDNF mRNA levels and BDNF protein levels in ED KIV mice in this study and in our previous study (Jha et al., 2016). Defective promoter IV-driven BDNF transcription has been observed in the frontal cortex under early-life maltreatment (Roth et al., 2009). Our findings suggest that EET implemented at early life, but not at the later time, is critical to compensate for promoter IV-BDNF deficiency by activating alternative promoters in the frontal cortex.
Furthermore, our results demonstrated that EET effects on promoter-specific BDNF induction in both the hippocampus and frontal cortex were mostly sustained only at early-life development in promoter IV-BDNF deficiency. This result highlights early life as a critical period to induce lasting BDNF transcription possibly due to the homeostatic compensational effects in promoter IV defect, while the EET effects may already be maximal in the normal condition (ceiling effects).
5. Why is EET at early-life development more effective than EET at later life in inducing lasting BDNF transcription in BDNF deficiency?
The exact reason is unknown, but one possibility is due to epigenetic programming (e.g., DNA and histone modifications) which is more drastic, flexible (Lister et al., 2013), and long-lasting (Champagne & Curley, 2009, Lister et al., 2013, Meaney & Szyf, 2005, Weaver et al., 2004) during early-life development than in adulthood. Indeed, a dynamic demethylation at BDNF promoters I, II, IV, and VI has been reported from embryonic pallium to the neonate hippocampus at postnatal day 7 (Dennis & Levitt, 2005). The dynamic epigenetic programming may change BDNF transcriptional regulation and allow animals to adapt to the exposed environment by changing neuronal plasticity (Arai et al., 2009).
Additionally, early-life EET involves mother-pup/infant social enrichment. The importance of mother-infant relations for lasting physiological changes has been proposed for over 60 years in both human (Johnson et al., 1992, Winick et al., 1975) and animal models (Levine, 1957, Meaney & Szyf, 2005, Weininger, 1954). Interestingly, mother-pup social enrichment is increased by their brief (~15 min) separation, which can occur as mothers engage in EET (e.g., running). The brief separation increases the licking and grooming of pups upon reunion (Liu et al., 1997), and gentle touching increases the resistance of pups to physiological stress later in adulthood (Levine, 1957, Liu et al., 1997). The offspring of mothers exhibiting high levels of pup licking present increased BDNF expression in the hippocampus at postnatal day 8 (Liu et al., 2000), which likely involves epigenetic controls. Indeed, early social enrichments by communal nesting (where several mothers keep their litters together and share caregiving behavior) turns the epigenetic structure of the BDNF gene into a more active state in 5 months-old mice, increasing acetylated form of histone 3 at BDNF promoters I, IV, VI and VII in the hippocampus (Branchi et al., 2011). The early-life EET used in the present study includes social enrichment by communal nesting, in addition to physical exercise (running wheels) and cognitive stimulation (toys), and activated almost all BDNF promoters in both hippocampus and frontal cortex of KIV mice. It is possible that the mother-pup interaction provides additional epigenetic modifications on these promoters (I, IV, VI, VII) to complement the epigenetic changes induced by exercise [I, II, III, and IV (Adlard et al., 2005, Adlard et al., 2004, Gomez-Pinilla et al., 2011, Jha et al., 2011, Russo-Neustadt et al., 2000, Zajac et al., 2010)] and cognitive stimulation [IV (Bredy et al., 2007, Lubin et al., 2008, Takei et al., 2011)]. Epigenetic changes in these multiple BDNF promoters may last long to more effectively compensate for one promoter defect. Detailed epigenetic mechanisms involved in EET need to be further elucidated in the future. Targeting such mechanisms (e.g., epigenetic therapy on the promoter sites) that enable lasting BDNF transcription may become an alternative strategy to mimic lasting antidepressive and neurotrophic effects of EET, particularly for subjects with BDNF promoter IV defect.
6. Early-life EET as intervention for anhedonia and BDNF deficiency
Our data showed larger, long-lasting effects of early-life EET on anhedonia and BDNF induction in KIV mice, suggesting that EET was more effective in reversing the defects in behavior and BDNF levels, rather than in increasing these beyond the normal state. In real life, EET may be more effective for people in adverse environments (e.g., isolation and stress), rather than enhancing normal environments. Our findings also provide an important notion of when to implement EET for its maximal and lasting effects. While promoter IV-BDNF deficiency is implicated in depression (Dwivedi et al., 2003, Hing et al., 2012, Keller et al., 2010), epigenetic inactivation of promoter IV occurs by exposure to toxic environments, such as perinatal methylmercury (Onishchenko et al., 2008), early-life maltreatment (Roth et al., 2009), chronic social stress (Tsankova et al., 2006), and maternal low-protein diet (Marwarha et al., 2017). Defective promoter IV-driven BDNF transcription can transmit across generations via abnormal maternal behavior and depression (Roth et al., 2009). To break this vicious cycle of promoter IV defect and mother-infant maltreatment, early-life EET is likely critical and can reduce risk of depression later in life. Early-detection of BDNF deficiency, such as by measuring promoter IV inactivity and related biomarkers, would aid in initiating early-life EET.
7. Limitation and future directions
The EET in this study combined physical, mental, and social activity, so it is unclear which components are responsible for the anti-anhedonic effects and BDNF induction. The EET also covered birth through maturation, so the precise critical period (e.g., gestation, before weaning, childhood, or adolescence) for the most effective EET also remains unclear. Future studies can determine a more precise period in early life when EET provides the largest effects. Our hypothesis is that EET is most effective when neurons rapidly proliferate, migrate, and mature and before neurons and synapses start to decrease by apoptosis and pruning. This study only measured static levels of BDNF transcription. It will be of interest to elucidate how EET affects BDNF transcriptional activity upon stimuli (new environments, learning training, etc.) and how this affects the development of neuronal network connectivity and more broad behavioral phenotypes related to depression (e.g., flexible learning, anxiety, susceptibility to stress and drug addiction, etc.). Although KIV mice have been extensively backcrossed (>12 times) to C57BL/6, 129Sv genes in the close vicinity of the BDNF gene may remain in KIV mice and might have affected the EET responsivity.
8. Conclusion
The present study aimed to identify the life period when EET is most effective for anhedonia and BDNF gene induction. Our results demonstrated that EET was equally effective in reversing anhedonia and inducing hippocampal BDNF transcription, regardless of age and BDNF deficient conditions, but was more effective during ED in inducing frontal cortex BDNF transcription, specifically in promoter IV-BDNF deficiency. Almost all BDNF promoters contributed to BDNF gene induction, where promoters I and IV in the WT hippocampus and almost all promoters in the KIV frontal cortex showed age and genotype dependency. Most promoter activity and anti-anhedonic effects persisted when EET was provided during early life, particularly in promoter IV-BDNF deficiency. The results suggest that EET is a universal intervention against anhedonia across ages and that early-life EET is a strategy for lasting intervention against anhedonia and BDNF deficiency.
Supplementary Material
Acknowledgments
We thank Joshua Mastin, Sean Duke, and Afshin Paydar for technical support. NIH grants to K.S. (MH102445, MH105567).
Footnotes
Conflict of Interest The authors declare no conflict of interest.
Supplementary information is available.
References
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai JA, Li S, Hartley DM, Feig LA. Transgenerational rescue of a genetic defect in long-term potentiation and memory formation by juvenile enrichment. J Neurosci. 2009;29:1496–1502. doi: 10.1523/JNEUROSCI.5057-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Cheng P, Monteggia LM. Gender-specific impact of brain-derived neurotrophic factor signaling on stress-induced depression-like behavior. Biol Psychiatry. 2009;66:84–90. doi: 10.1016/j.biopsych.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EL, Rosenzweig MR, Diamond MC. Rat brain: effects of environmental enrichment on wet and dry weights. Science. 1969;163:825–826. doi: 10.1126/science.163.3869.825. [DOI] [PubMed] [Google Scholar]
- Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van OJ, Lesch KP, Lanfumey L, Steinbusch HW, Kenis G. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry. 2012;17:584–596. doi: 10.1038/mp.2011.107. [DOI] [PubMed] [Google Scholar]
- Branchi I, Karpova NN, D’Andrea I, Castren E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett. 2011;495:168–172. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the brain. Oxford University Press; New York, NY: 2006. [Google Scholar]
- Castren E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci Biobehav Rev. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Brandwein C, Vogt MA, Dormann C, Hellweg R, Gass P. Nature vs. nurture: can enrichment rescue the behavioural phenotype of BDNF heterozygous mice? Behav Brain Res. 2008;192:254–258. doi: 10.1016/j.bbr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Brandwein C, Gass P. Altering BDNF expression by genetics and/or environment: impact for emotional and depression-like behaviour in laboratory mice. Neurosci Biobehav Rev. 2011;35:599–611. doi: 10.1016/j.neubiorev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis KE, Levitt P. Regional expression of brain derived neurotrophic factor (BDNF) is correlated with dynamic patterns of promoter methylation in the developing mouse forebrain. Brain Res Mol Brain Res. 2005;140:1–9. doi: 10.1016/j.molbrainres.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Dimeo F, Bauer M, Varahram I, Proest G, Halter U. Benefits from aerobic exercise in patients with major depression: a pilot study. Br J Sports Med. 2001;35:114–117. doi: 10.1136/bjsm.35.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Flurkely K, Currer JM, Harrison DE. Mouse Models in Aging Research. Academic Press; 2007. [Google Scholar]
- Fuchikami M, Morinobu S, Kurata A, Yamamoto S, Yamawaki S. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. Int J Neuropsychopharmacol. 2009;12:73–82. doi: 10.1017/S1461145708008997. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2010;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2011;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The effects of early experience on problem-solving at maturity. Am Psychol. 1947;2:306–307. [Google Scholar]
- Hing B, Davidson S, Lear M, Breen G, Quinn J, McGuffin P, MacKenzie A. A polymorphism associated with depressive disorders differentially regulates brain derived neurotrophic factor promoter IV activity. Biol Psychiatry. 2012;71:618–626. doi: 10.1016/j.biopsych.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui-Yamamoto C, Yamamoto T, Ueda K, Nakatsuka M, Kumabe S, Inui T, Iwai Y. Taste preference changes throughout different life stages in male rats. PLoS One. 2017;12:e0181650. doi: 10.1371/journal.pone.0181650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jax. https://www.jax.org/research-and-faculty/research-labs/the-harrison-lab/gerontology/life-span-as-a-biomarker.
- Jha S, Dong B, Sakata K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Translational Psychiatry. 2011;1:e40. doi: 10.1038/tp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha S, Dong BE, Xue Y, Delotterie DF, Vail MG, Sakata K. Antidepressive and BDNF effects of enriched environment treatment across ages in mice lacking BDNF expression through promoter IV. Transl Psychiatry. 2016;6:e896. doi: 10.1038/tp.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Miller LC, Iverson S, Thomas W, Franchino B, Dole K, Kiernan MT, Georgieff MK, Hostetter MK. The health of children adopted from Romania. JAMA. 1992;268:3446–3451. [PubMed] [Google Scholar]
- Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, Sacchetti S, Lembo F, Angiolillo A, Jovanovic N, Pisanti F, Tomaiuolo R, Monticelli A, Balazic J, Roy A, Marusic A, Cocozza S, Fusco A, Bruni CB, Castaldo G, Chiariotti L. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry. 2010;67:258–267. doi: 10.1001/archgenpsychiatry.2010.9. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Tamura R, Hareyama N, Imai S, Narita M, Torigoe K, Niikura K, Takeshima H, Ando T, Igarashi K, Kanno J, Ushijima T, Suzuki T, Narita M. Hippocampal epigenetic modification at the brain-derived neurotrophic factor gene induced by an enriched environment. Hippocampus. 2011;21:127–132. doi: 10.1002/hipo.20775. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, Hu S, Wu JC, Rao A, Esteller M, He C, Haghighi FG, Sejnowski TJ, Behrens MM, Ecker JR. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu QR, Walther D, Drgon T, Polesskaya O, Lesnick TG, Strain KJ, de Andrade M, Bower JH, Maraganore DM, Uhl GR. Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson’s Disease. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:93–103. doi: 10.1002/ajmg.b.30109. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatynska E, Steinbusch HW, Redkozubova O, Bolkunov A, Kubatiev A, Yeritsyan NB, Vignisse J, Bachurin S, Strekalova T. Anhedonic-like traits and lack of affective deficits in 18-month-old C57BL/6 mice: Implications for modeling elderly depression. Exp Gerontol. 2012;47:552–564. doi: 10.1016/j.exger.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Schloesser RJ, Jimenez DV, Weinberger DR, Lu B. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol Brain. 2011;4:11. doi: 10.1186/1756-6606-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsen EW. Benefits of exercise for the treatment of depression. Sports Med. 1990;9:380–389. doi: 10.2165/00007256-199009060-00006. [DOI] [PubMed] [Google Scholar]
- Marwarha G, Claycombe-Larson K, Schommer J, Ghribi O. Maternal low-protein diet decreases brain-derived neurotrophic factor expression in the brains of the neonatal rat offspring. J Nutr Biochem. 2017;45:54–66. doi: 10.1016/j.jnutbio.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard KR, Hill JL, Calcaterra NE, Palko ME, Kardian A, Paredes D, Sukumar M, Adler BD, Jimenez DV, Schloesser RJ, Tessarollo L, Lu B, Martinowich K. Functional Role of BDNF Production from Unique Promoters in Aggression and Serotonin Signaling. Neuropsychopharmacology. 2016;41:1943–1955. doi: 10.1038/npp.2015.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsis M, Timmusk T, Arenas E, Persson H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci U S A. 1993;90:8802–8806. doi: 10.1073/pnas.90.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Neidl R, Schneider A, Bousiges O, Majchrzak M, Barbelivien A, de Vasconcelos AP, Dorgans K, Doussau F, Loeffler JP, Cassel JC, Boutillier AL. Late-Life Environmental Enrichment Induces Acetylation Events and Nuclear Factor kappaB-Dependent Regulations in the Hippocampus of Aged Rats Showing Improved Plasticity and Learning. J Neurosci. 2016;36:4351–4361. doi: 10.1523/JNEUROSCI.3239-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castren E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J Neurochem. 2008;106:1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Reiner A, Wang HB, Del Mar N, Sakata K, Yoo W, Deng YP. BDNF may play a differential role in the protective effect of the mGluR2/3 agonist LY379268 on striatal projection neurons in R6/2 Huntington’s disease mice. Brain Res. 2012;1473:161–172. doi: 10.1016/j.brainres.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR. Environmental complexity, cerebral change, and behavior. Am Psychol. 1966;21:321–332. doi: 10.1037/h0023555. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Sakata K. Brain Derived Neurotrophic Factor and Major Depression. In: López-Muñoz F, editor. Neurobiology of Depression, Frontiers in Neuroscience. CRC press; Florida: 2011. pp. 391–417. [Google Scholar]
- Sakata K. Brain-Derived Neurotrophic Factor for Depression Therapeutics. Journal of Pharmacology and Therapeutics. 2014;2:1–10. [Google Scholar]
- Sakata K, Duke SM. Lack of BDNF expression through promoter IV disturbs expression of monoamine genes in the frontal cortex and hippocampus. Neuroscience. 2014;260:265–275. doi: 10.1016/j.neuroscience.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Sakata K, Jin L, Jha S. Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav. 2010;9:712–721. doi: 10.1111/j.1601-183X.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- Sakata K, Martinowich K, Woo NH, Schloesser RJ, Jimenez DV, Ji Y, Shen L, Lu B. Role of activity-dependent BDNF expression in hippocampal-prefrontal cortical regulation of behavioral perseverance. Proc Natl Acad Sci U S A. 2013a;110:15103–15108. doi: 10.1073/pnas.1222872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K, Mastin JR, Duke SM, Vail MG, Overacre AE, Dong BE, Jha S. Effects of antidepressant treatment on mice lacking brain-derived neurotrophic factor expression through promoter IV. Eur J Neurosci. 2013b;37:1863–1874. doi: 10.1111/ejn.12148. [DOI] [PubMed] [Google Scholar]
- Sakata K, Overacre AE. Promoter IV-BDNF deficiency disturbs cholinergic gene expression of CHRNA5, CHRM2, and CHRM5: effects of drug and environmental treatments. J Neurochem. 2017;143:49–64. doi: 10.1111/jnc.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Segovia G, del Arco A, Mora F. Environmental enrichment, prefrontal cortex, stress, and aging of the brain. J Neural Transm. 2009;116:1007–1016. doi: 10.1007/s00702-009-0214-0. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. Journal of Neuroscience. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, Prieto GA, Petrosyan A, Loertscher BM, Dieskau AP, Overman LE, Cotman CW. H3K9me3 Inhibition Improves Memory, Promotes Spine Formation, and Increases BDNF Levels in the Aged Hippocampus. Journal of Neuroscience. 2016;36:3611–3622. doi: 10.1523/JNEUROSCI.2693-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, Kuraishi Y, Tsuda M. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem. 2000;275:17269–17275. doi: 10.1074/jbc.M909538199. [DOI] [PubMed] [Google Scholar]
- Takei S, Morinobu S, Yamamoto S, Fuchikami M, Matsumoto T, Yamawaki S. Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. J Psychiatr Res. 2011;45:460–468. doi: 10.1016/j.jpsychires.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tordoff MG. Taste solution preferences of C57BL/6J and 129X1/SvJ mice: influence of age, sex, and diet. Chem Senses. 2007;32:655–671. doi: 10.1093/chemse/bjm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weininger O. Physiological damage under emotional stress as a function of early experience. Science. 1954;119:285–286. doi: 10.1126/science.119.3087.285. [DOI] [PubMed] [Google Scholar]
- WHO. DEPRESSION AND OTHER COMMON MENTAL DISORDERS: GLOBAL HEALTH ESTIMATES. 2017 http://apps.who.int/iris/bitstream/10665/254610/1/WHO-MSD-MER-2017.2-eng.pdf?ua=1,WHO/MSD/MER/2017.2012.
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Winick M, Meyer KK, Harris RC. Malnutrition and environmental enrichment by early adoption. Science. 1975;190:1173–1175. doi: 10.1126/science.1198103. [DOI] [PubMed] [Google Scholar]
- Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac MS, Pang TY, Wong N, Weinrich B, Leang LS, Craig JM, Saffery R, Hannan AJ. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington’s disease mice. Hippocampus. 2010;20:621–636. doi: 10.1002/hipo.20658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.