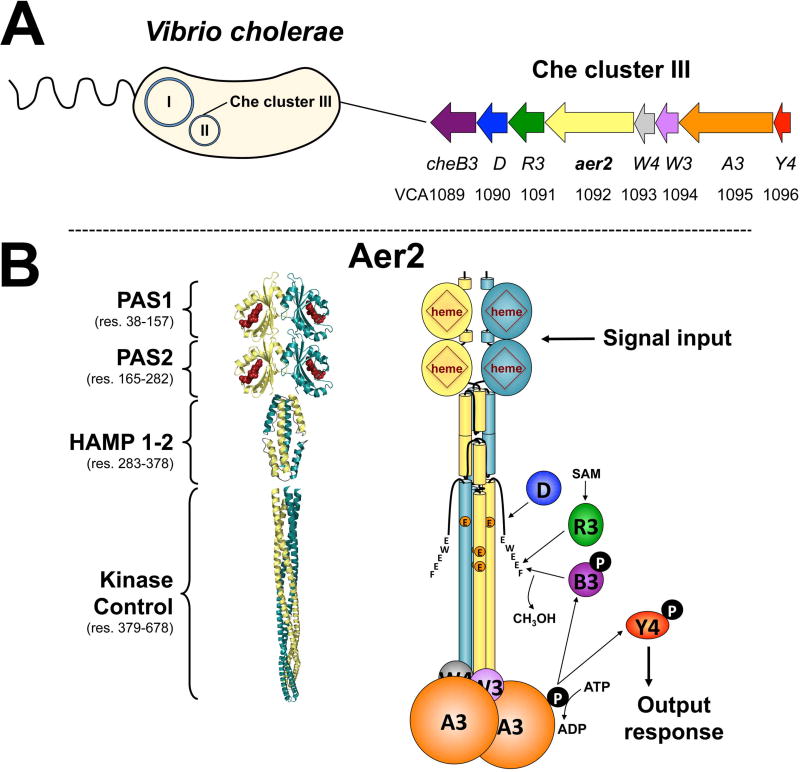
Fig. 1. V. cholerae chemosensory cluster III and the proposed structure of VcAer2.
A. V. cholerae chemosensory (Che) cluster III resides on chromosome II and encodes a complete chemosensory system. This includes a chemoreceptor (Aer2), a histidine kinase (CheA3), two coupling proteins (CheW3 and W4), a response regulator (CheY4), and three adaptation enzymes (CheR3, D and B3).
B. The proposed structure of a VcAer2 dimer (left) and the cluster III chemosensory pathway (right). VcAer2 is predicted to contain two N-terminal PAS domains (PAS1 and PAS2), a di-HAMP unit (HAMP 1–2), and a kinase control module that is typical of methyl-accepting chemoreceptors [containing three putative methylation sites: EEE, residues 414, 421 and 603, and a C-terminal pentapeptide sequence (EWEEF) for the binding of adaption enzymes]. In the left panel, PAS1 and PAS2 are modeled on the structure of P. aeruginosa Aer2 PAS [PDB code: 4HI4, (Airola et al., 2013a)], the di-HAMP unit is modeled on the structure of P. aeruginosa Aer2 HAMP 2–3 [PDB code: 3LNR, (Airola et al., 2010)], and the kinase control module is modeled on the structure of MCP1143C [PDB code: 2CH7, (Park et al., 2006)]. VcAer2 signaling is proposed to activate CheA3 autophosphorylation, which in turn phosphorylates CheY4, which purportedly regulates the response from the cluster III chemosensory system. VcAer2 signaling is modulated by the adaptation enzymes CheR3, D and B3, which bind the C-terminal pentapeptide EWEEF and/or the kinase control module to modify the methylation status of VcAer2. Abbreviation: SAM, S-adenosylmethionine.