Abstract
Perivascular adipose tissue (PVAT) has the capacity to secrete vasoactive mediators with the potential to regulate vascular function. Given its location adjacent to the vasculature, PVAT dysfunction may be part of the pathophysiology of cardiovascular diseases. To study the mechanisms of PVAT dysfunction, several adipogenic models have been proposed. However, these approaches do not adequately reflect PVAT adipocyte phenotypes variability that depends on their anatomical location. Despite PVAT importance in modulating vascular function, to date, there is not a depot-specific adipogenic model for PVAT adipocytes. We present a model that uses coculturing of PVAT stromal vascular fraction derived preadipocytes with primary adipocytes isolated from the same PVAT. Preadipocytes were isolated from thoracic aorta PVAT and mesenteric resistance artery PVAT (mPVAT). Upon confluency, cells were induced to differentiate for 7 and 14 days using a standard protocol (SP) or standard protocol cocultured with primary adipocytes isolated from the same adipose depots (SPA) for 96, 120, and 144 h. SPA reduced the time for differentiation of stromal vascular fraction derived preadipocytes and increased their capacity to store lipids compared with SP as indicated by lipid accumulation, lipolytic responses, gene marker profile expression, and adiponectin secretion. The coculture system improved adipogenesis efficiency by enhancing lipid accumulation and reducing the time of induction, therefore, is a more efficient method compared to SP alone.
Keywords: Perivascular adipose tissue, PVAT, Adipogenesis, Coculture
Background
Perivascular adipose tissue (PVAT) is an important modulator of vascular function given its anatomical location and its capacity to secrete vasoactive molecules including proteins and lipids (Watts et al. 2013). PVAT’s capacity to modulate vascular function is affected in part by the phenotype of its adipocytes, white or brown, that largely depends on its anatomical location (Padilla et al. 2013). PVAT surrounding the thoracic aorta has a brown phenotype while PVAT surrounding the small mesenteric resistance vessels is predominantly white (Contreras et al. 2016). This anatomical distribution of PVAT phenotypes is observed not only in rodents, but also in canine models for cardiovascular research (Payne et al. 2009) and in humans (Wei et al. 2015). White adipocytes function largely as an energy storing unit. In contrast, brown adipocytes are thermogenic given its expression of the heat generating mitochondrial uncoupling protein 1 (UCP1) (Rajsheker et al. 2010; Sanchez-Gurmaches et al. 2016). During metabolic diseases resulting from excessive energy intakes, such as obesity and metabolic syndrome, adipose tissue is expanded by hyperplasia and hypertrophy (Wang et al. 2013). These processes allow for increased cell number with greater lipid storage capacity within PVAT, however when dysregulated, lead to alterations in its capacity to modulate vessel function predisposing to cardiovascular disease (Lian and Gollasch 2016).
The study of PVAT adipocyte biology has relied on adipogenic cell lines including 3T3-L1 (Ismail et al. 2017; Shoham and Gefen 2011), 3T3-F442A, and OP9 (Ruiz-Ojeda et al. 2016). However, these models do not fully represent the phenotype variations between PVAT depots and lack the unique genetic and epigenetic profiles of PVAT derived preadipocytes. The latter characteristic is of especial importance given the current lack of specific markers of PVAT adipocytes (Ouwens et al. 2010). Furthermore, adipogenic models of visceral adipose tissue, where mesenteric PVAT (mPVAT) is located, and thoracic PVATs have low adipocyte differentiation rates compared to subcutaneous depots (Chatterjee et al. 2009; Tchkonia et al. 2002, 2006). Despite its importance in modulating blood pressure and vascular function, there is not a depot-specific adipogenic model for PVATs that faithfully represents their specific phenotype and functions. This would allow for further study on PVAT biology in healthy and disease states. The objective of this study was to create a depot-specific adipogenic model for visceral and thoracic PVATs. We present a coculture approach for stromal vascular fraction (SVF) derived preadipocytes differentiation by exposing them to homologous primary adipocytes isolated from the same PVAT depots. Our study shows that this method provides a greater adipogenic response in a shorter period of time when compared to a pharmacological induction protocol alone.
Methods
Ethics statement
All animal use described here followed guidelines established by the Institutional Animal Care and Use Committee (IACUC) of Michigan State University.
Animals and tissue processing
Male Sprague Dawley rats of 8–10 weeks of age were euthanized using 70 mg/kg of pentobarbital delivered via an intraperitoneal injection. PVAT from small mesenteric resistance vessels (mPVAT) and the thoracic aorta (aPVAT), as well as gonadal (GON) adipose depot, used as a visceral non PVAT control, were collected in Krebs Ringer bicarbonate buffer (KRBB) supplemented with HEPES (10 mM, pH = 7.4; Thermo Fisher, Waltham, MA, USA) as previously described (Contreras et al. 2016; Thelen et al. 2017). Adipose tissues (50 mg) were digested with 1 mL collagenase type I (Worthington Biochemical, Lakewood, NJ, USA) solution (1 mg/mL) and then centrifuged (800 g, 10 min, 20 °C) to separate the primary adipocytes from the SVF. Primary adipocytes were washed in 1 mL of KRBB with 4% bovine serum albumin, centrifuged (400 g, 20 min, 20 °C), and then retained for use in transwell inserts (Greiner Bio-One, Kremsmünster, Austria) for inductions. The SVF was sequentially filtered through 100 and 40 µm cell strainers (Thermo Fisher). The filtrate was then centrifuged (800 g, 10 min, 20 °C) and resulting cell pellet was resuspended and incubated in erythrocyte lysis buffer for 3 min at 20 °C (Biolegend, San Diego, CA, USA). After an additional centrifugation (800 g, 10 min, 20 °C), pelleted cells were resuspended in preadipocyte medium containing 10% fetal bovine serum (Corning, Corning, NY, USA), 44.05 mM sodium bicarbonate (Thermo Fisher), 100 µm ascorbic acid (Sigma-Aldrich, St Louis, MO, USA), 33 µm biotin (Sigma-Aldrich), 17 µm pantothenate (Sigma-Aldrich), 1% l-glutamine (Thermo Fisher), 1% antibiotic/antimycotic solution (Thermo Fisher), and 2% HEPES. Cells were then incubated at 37 °C 5%CO2. To eliminate immune cells, preadipocyte populations were expanded for two serial passages in culture flasks (Nest Biotechnology, Rahway, NJ, USA). Two serial passages were shown to select for fibroblast-like cell populations expressing adipocyte progenitor cell markers including CD34 (Mitchell et al. 2006). At the same time, even just a single passage reduces the populations of immune cells including macrophages, granulocytes, and lympohcytes to levels that are not detectable by flow cytometry assays (Brzoska et al. 2005).
Standard induction protocol (SP)
After two serial passages, preadipocytes were seeded in 6 well and 24 well plates (Corning) at a concentration of 20,000 cells/cm2 and allowed to reach confluency. Preadipocytes were then induced to differentiate using an adipocyte induction medium containing 10% fetal bovine serum (Corning), 5 µg/mL insulin (Sigma-Aldrich), 200 pmol/L T3 (Sigma-Aldrich), and 0.5 µM rosiglitazone (Cayman Chemical, Ann Arbor, MI, USA). The following supplements from Sigma-Aldrich were used for the first 48 h of culture: 0.5 mM IBMX and 0.25 µM Dexamethasone. Medium was changed every 48 h. SP protocol is summarized in Fig. 1.
Fig. 1.
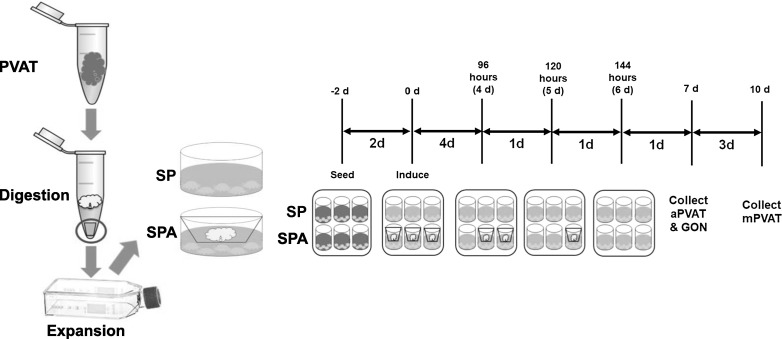
Schematic diagram of stromal vascular fraction (SVF) derived preadipocyte collection and adipogenic induction. Perivascular adipose tissues (PVAT) are collected and digested with collagenase and then expanded in tissue flasks. After 2 days of expansion, SVF are plated in multi-well plates and induced to differentiate in the absence (SP) or presence (SPA) of primary adipocytes. Thoracic aorta preadipocytes (aPVAT) and gonadal (GON, as non PVAT controls) are cultured for 7 or 14 days. Mesenteric PVAT are cultured for 10 or 14 days
Coculture (SPA)
Expanded preadipocytes populations were seeded in 6 well and 24 well plates (Corning) at a concentration of 20,000 cells/cm2 and allowed to proliferate to confluency. Preadipocytes were then induced as in SP but a 0.4 µm transwell insert (Greiner Bio-One) containing 900 cells/cm2 primary adipocytes over the preadipocytes was also included. Medium was changed every 48 h. SPA protocol is summarized in Fig. 1.
Culture optimization
To determine optimal conditions for SPA, preadipocytes were cultured in 24 well plates for a total of 7 days (aPVAT, mPVAT, GON) or 10 days (mPVAT). Inserts of primary adipocytes were removed at 96, 120 and 144 h post induction and then preadipocytes were allowed to continue its differentiation process (Fig. 1). At the end of the differentiation period (d7 for aPVAT and GON, and d10 for mPVAT), triacylglyceride (TAG) accumulation was analyzed using the AdipoRed™ assay (Lonza, Basel, Switzerland). AdipoRed™ results are reported as the relative fluorescence units ratio adipocyte:preadipocyte. Live cell counts of primary adipocytes were performed at 0 and 144 h of coculture using Trypan Blue (Sigma-Aldrich). Preadipocytes were also induced to differentiate using Standard Induction Protocol (SP) alone for the same differentiation period as their cocultured pairs (d7 for aPVAT, mPVAT and GON, and d10 for mPVAT) and for 14 days as a control for SP protocols (Fig. 1).
Adipogenesis efficiency
Expanded preadipocyte populations were seeded in a glass bottom 24 well plate (Corning) at a concentration of 20,000 cells/cm2, allowed to proliferate until confluency, and then induced to differentiate by either SP or SPA methods described above. After differentiation, cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) and then stained with NucBlue™ and HCS LipidTox™ (Life Technologies, Carlsbad, CA, USA) to identify nuclei and triacylglycerides (TAG), respectively. Cells were imaged using an Olympus FluoView FV1000 filter-based Confocal Laser Scanning Microscope paired with Olympus FV10-ASW software (Olympus, Waltham, MA, USA). Efficiency was determined by counting the number of differentiated, containing at least one lipid droplet, versus non-differentiated cells. ImageJ software was used to measure triacylglyceride (TAG) accumulation.
Functional assays
Cultured adipocytes differentiated by either SP for 7 or 14 days or SPA for 7 days were removed from 6-well plates (Corning) using Trypsin (Thermo Fisher). Adipocytes were then seeded in triplicate at 100,000 cells/well in black wall 96-well plates (Nunc, Roskilde, Denmark). For lipolysis assays, cells were serum starved for 4 h and then stimulated with the beta-adrenergic receptor agonist isoproterenol (1 µM, Sigma-Aldrich). After 2 h of incubation, the supernatant was removed, and glycerol content was measured (Glycerol Assay, MAK117, Thermo Fisher). The CyQUANT Assay (Life Technologies, Carlsbad, CA, USA) was used to determine cell number in each well post functional analysis. Adiponectin secretion into culture medium was quantified using a rat-specific ELISA kit (KRP0041, Thermo Fisher).
Gene expression analysis
RNA was extracted from cells using the Maxwell® RSC simplyRNA cells Kit (Cat# AS1390) and a Maxwell® RSC Instrument according to manufacturer instructions (Promega, Madison, WI, USA). Cultured cells were harvested in 200 μL of lysis buffer and then vortexed vigorously for 15 s. The sample was then processed on the Maxwell® RSC Instrument using simplyRNA cartridges containing 10 μL of blue DNase I solution in each designated well. After extraction, samples were eluted in nuclease-free water. Purity, concentration, and integrity of mRNA were evaluated using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and an Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). For all samples, the 260:280 nm ratio was between 1.9 and 2.1 and the RNA integrity number > 7. Conversion to cDNA was performed using the Applied Biosystems High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). All quantitative qPCR assays were performed using SYBR (ABsolute Blue QPCR SYBR; Thermo Scientific). Adiponectin (Adipoq), perilipin 1 (Plin1), uncoupling protein 1 (Ucp1), PR domain containing 16 (Prdm16), and Iodothyronine Deiodinase 2 (Dio2) cDNA were amplified using forward and reverse primer sequences described in Contreras et al. (2016). Fatty acid binding protein 4 (Fabp4, NM_053365.1) cDNA was amplified using forward primer 5′- GTCCT GGTACATGTGCAGAA-3′ and reverse primer 5′- CTCTTGTAGAAGTCACGCCT-3′. A non-reverse transcriptase control was run to ensure that genomic DNA was not being amplified. Reference genes were selected using GeNorm from a pool of 4 candidates (Actb, B2m, Gapdh, Rps9). Expression of genes of interest was normalized against the geometric mean of Actb, B2m and Rps9 as described (Contreras et al. 2017).
Statistical analysis
Data are reported as mean ± SEM. Data were analyzed by one- or two-way ANOVA or Proc Mixed (JMP Software, SAS Institute, Cary, NC, USA). Post hoc comparisons were performed using Tukey’s test. Statistical significance was set at P ≤ 0.05.
Results
To standardize SPA conditions for the different SVF-derived preadipocytes, we first optimized the time of primary adipocyte coincubation. Confluent preadipocytes were induced to differentiate for 7 days by incubation in SP medium. Starting on day 1 of the differentiation process, preadipocytes were exposed to primary adipocytes from the same site for 96, 120, and 144 h. After co-incubation, cells were further maintained in SP medium until day 7. Cells from aPVAT and GON exhibited increased TAG accumulation at day 7 when co-incubated for 144 and 120 h with primary adipocytes, respectively (Fig. 2A). However, TAG accumulation in cells from mPVAT did not increase significantly at the end of the differentiation time (Fig. 2A). The total differentiation time for mPVAT was then increased from 7 to 10 days. In mPVAT preadipocytes, 10 days of total culture time (Fig. 2B) and 120 h of primary adipocyte incubation increased TAG accumulation compared to SP (Fig. 2C). To investigate the effect of SPA conditions on primary adipocytes viability, a Trypan Blue staining was performed at 0 and 144 h of cell culture. There was no difference between the viability of primary adipocytes at either time point (Fig. 2D). Once culture conditions were optimized (Table 1), cells from each depot were cultured to perform functional assays and gene transcription analyses.
Fig. 2.
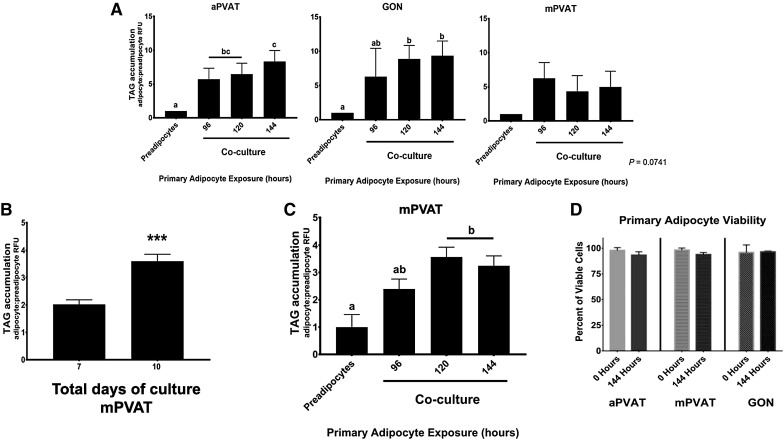
Coculture with primary adipocytes enhances the accumulation of TAG in stromal vascular fraction (SVF) derived preadipocytes without affecting the viability of primary adipocytes. A TAG accumulation in SVF-derived preadipocytes from aortic PVAT (aPVAT), gonadal adipose (GON), and mesenteric PVAT (mPVAT) after 7 days of differentiation. During this period, cells were cocultured with primary adipocytes for 96, 120, or 144 h. TAG was assessed using AdipoRed™ assay. Data are represented as the ratio of adipocytes (day 7): preadipocytes (day 0) relative fluorescence units (RFU) ± SEM. Significant differences are indicated by letters a, b (P < 0.05, n = 4). B TAG accumulation is enhanced in mPVAT SVF-derived preadipocytes after coculture with primary mPVAT adipocytes for 120 h and 10 days of total culture time compared to 7 days total culture time. Data are represented as the ratio of adipocytes (day 7 or 10): preadipocytes (day 0) relative fluorescence units (RFU) ± SEM. Significant differences are indicated by *** (P < 0.001, n = 4). C TAG accumulation in SVF-derived preadipocytes from mPVAT after 10 days of total culture time. During this period, cells were cocultured with primary adipocytes for 96, 120, or 144 h. Data are represented as the ratio of adipocytes (day 10): preadipocytes (day 0) relative fluorescence units (RFU) ± SEM. Significant differences are indicated by letters a, b (P < 0.05, n = 4). D Coculture conditions do not affect the viability of primary adipocytes up to 144 h post-collection. Percentage of live primary adipocytes at 0 and 144 h of coculture. Data are represented as mean percent viability ± SEM (n = 4)
Table 1.
Optimal conditions for differentiation of stromal vascular fraction derived preadipocytes in coculture with primary isolated adipocytes (SPA)
| Site | Primary adipocyte exposure (h) | Total differentiation time (days) |
|---|---|---|
| Aortic PVAT | 144 | 7 |
| Mesenteric PVAT | 120 | 10 |
| Gonadal adipose | 120 | 7 |
Exposure of preadipocytes to primary adipocytes and total differentiation times as determined by time course trial and assessing triacylglyceride accumulation using the AdipoRed™ assay
We next evaluated the efficiency of the different adipogenic protocols using confocal microscopy. Protocols included SPA (with primary adipocytes) for 7 days (SPA7), SP (no primary adipocytes) for 7 (SP7) and 14 (SP14) days. SVF-derived preadipocytes from aPVAT and GON exhibited a higher adipogenic efficiency when induced in SPA7 or SP14 compared to SP7 (Fig. 3A). SVF-derived preadipocytes from mPVAT had a higher adipogenic efficiency in SPA7 compared to SP7 and SP14 (Fig. 3A). The adipogenic efficiency was reflected in TAG accumulation. For aPVAT and GON, all induction protocols increased TAG accumulation compared to preadipocytes before induction (Fig. 3B, C). In mPVAT, TAG accumulation was higher in adipocytes differentiated by SPA7 compared to SP7 and SP14 (Fig. 3B, C).
Fig. 3.
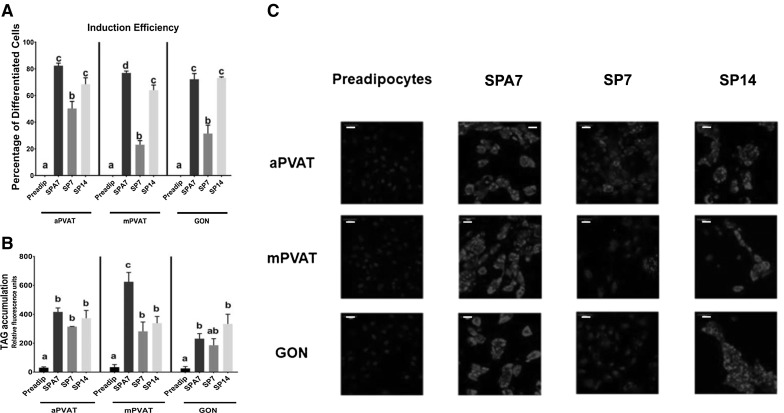
Coculture with primary adipocytes accelerates accumulation of TAG in stromal vascular fraction (SVF) derived preadipocytes and increases adipogenic efficiency compared to those induced with standard protocols. SVF derived preadipocytes from aortic (aPVAT), mesenteric (mPVAT), perigonadal (GON) depots were stimulated to differentiate by coculture with primary adipocytes (SPA7) or standard pharmacological induction for 7 (SP7) and 14 (SP14) days. A Induction efficiency measured as % of cells with a lipid droplet. Values are percentage of differentiated cells (cells with lipid droplets/total number of cells) ± SEM. Significant differences are indicated by letters a, b, c, d (P < 0.05, n = 3–5). B Differentiated adipocytes were stained with NucBlue™ and HCS LipidTox™ to identify nuclei and TAG, respectively. Values are mean TAG fluorescence per adipocyte ± SEM. Significant differences are indicated by letters a, b, c (P < 0.05, n = 4). C Representative (n = 3–5) high-resolution images of aPVAT, mPVAT, GON as preadipocytes and after differentiation by SPA7, SP7, and SP14. Cells’ nuclei were stained with NucBlue™ (blue) and triacylglycerides were stained with HCS LipidTOX™ (red). Scale bars represent 50 microns. (Color figure online)
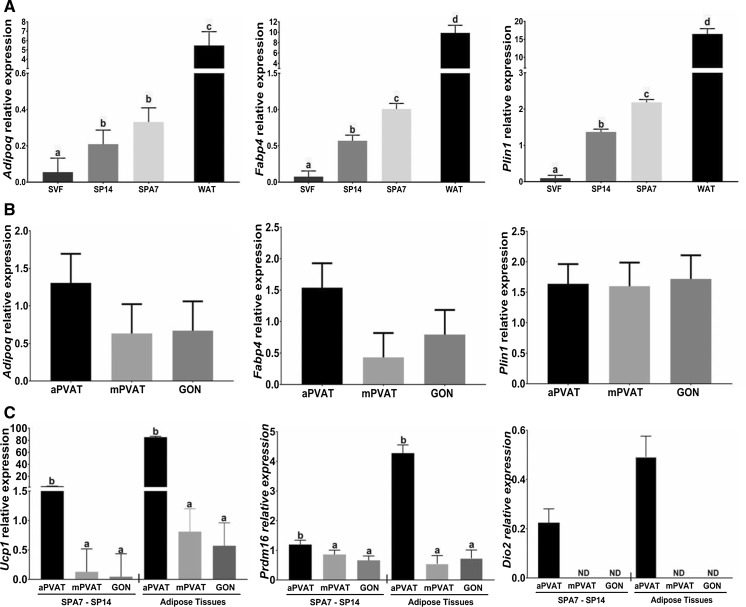
Adipocytes differentiated by SPA7 and SP14 and the stromal vascular fraction (SVF) from aPVAT, mPVAT, and GON depots were collected to extract RNA and assess the expression of gene markers of adipogenesis and adipocyte function using RT-qPCR. When evaluating the treatment effect, we observed that SP14 and SPA7 increased the expression of the gene Adipoq, that encodes adiponectin, compared to SVF in both PVAT depots and GON (Fig. 4A). In aPVAT, mPVAT, and GON, transcription of Fabp4 (encoding fatty acid binding protein 4) and Plin1 (encoding perilipin 1) was higher in adipocytes differentiated by SP14 compared to SVF (Fig. 4A). Reflecting the higher adipogenicity of the SPA7 protocol, Fabp4 and Plin1 expression in preadipocytes induced by this treatment was higher than with the SP14 protocol (Fig. 4A). We next evaluated the effect of anatomical site (i.e. aPVAT, mPVAT, GON) on the transcription of Adipoq, Fabp4, and Plin1 in adipocytes differentiated by SPA7 and SP14. No differences were detected among the sites in the transcription of these three adipogenic markers (Fig. 4B). Finally, we assessed the effect of anatomical site on the expression of brown phenotype-specific genes Ucp1, Prdm16, and Dio2. Adipocytes from aPVAT that were induced by SPA7 and SP14 had a higher expression of Ucp1 and Prdm16 compared to mPVAT and GON induced by the same methods (Fig. 4C). Additionally, the expression of a third brown phenotype marker, Dio2, was present in aPVAT adipocytes but not detected in mPVAT and GON (Fig. 4C). For comparison purposes we also evaluated the gene expression of these 3 brown phenotype markers in aPVAT, mPVAT, and GON tissues. Although the expression of these genes was higher in tissues than in cultured cells, it followed a similar expression pattern (Fig. 4C).
Fig. 4.
Coculture with primary adipocytes enhances the expression of adipogenic genes in stromal vascular fraction (SVF) derived preadipocytes compared to those induced with standard protocols. SVF-derived preadipocytes from aortic (aPVAT), mesenteric (mPVAT), perigonadal (GON) depots were stimulated to differentiate by coculture with primary adipocytes for 7 days (SPA7) or standard pharmacological induction for 14 days (SP14). A The effect of culture type on gene expression of adipogenic markers on cells from all sites (aPVAT, mPVAT, and GON). SVF before induction and gonadal white adipose tissue (WAT) were used as negative and positive controls. B Effect of anatomical site on the gene expression of adipogenic markers in differentiated adipocytes from SPA7 and SP14. C Effect of anatomical site on the expression of brown adipocyte markers in differentiated adipocytes from SPA7 and SP14 and adipose tissues collected from aPVAT, mPVAT and GON. Gene expression was evaluated by RT qPCR and normalized to Actb, B2m, and Rps29. Data are mean ± SEM (n = 6). ND = non detected. Significant differences are indicated by * and letters a, b, c, and d (P < 0.05), and *** (P < 0.001)
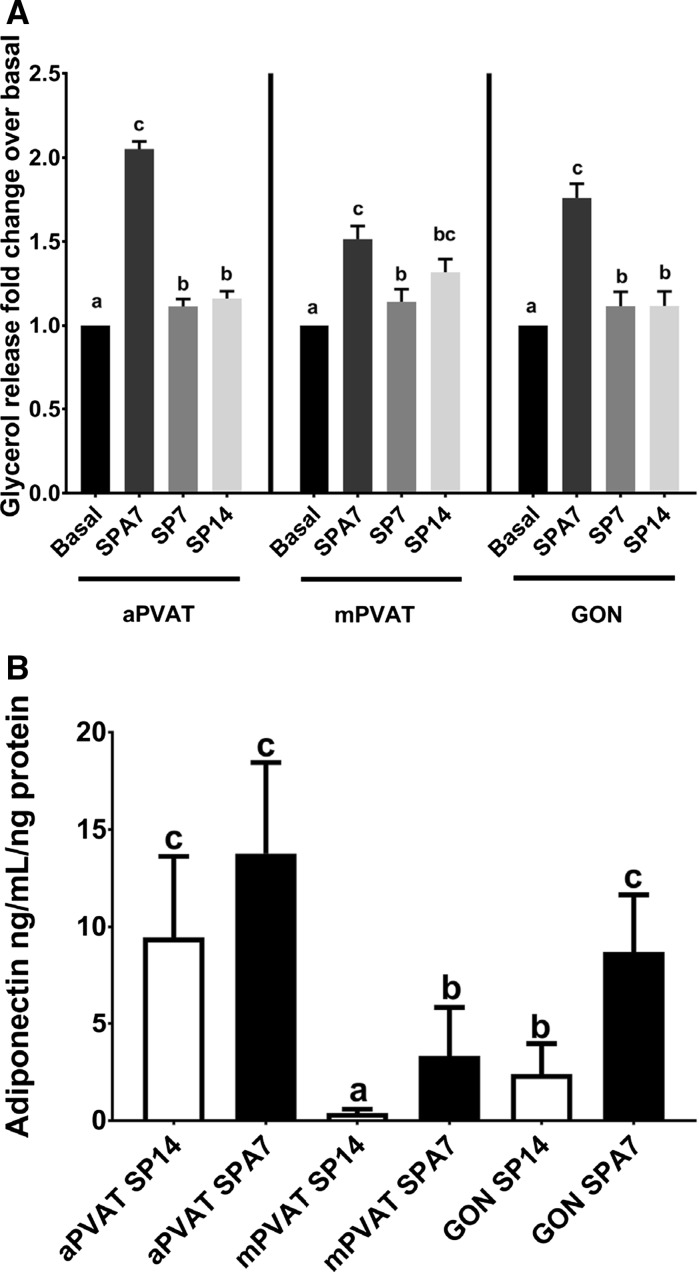
Adipocyte lipolytic response was assessed after stimulation with the β agonist, isoproterenol (1 µM) for 2 h. This dose and time were based on a standardized lipolysis protocol for rat adipocytes (Morimoto et al. 2001). Glycerol release was quantified and adjusted to the number of cells per tissue culture well, as determined by the fluorometric CyQUANT™ assay, compared to the basal state (i.e. unstimulated). For all fat sites, adipocytes differentiated in SPA7 conditions had higher lipolytic responses compared to those induced with SP7 and SP14 protocols (Fig. 5A). Since adiponectin is a canonical adipocyte protein with potent vasoactive activity, we evaluated the secretion of this adipokine by adipocytes from aPVAT, mPVAT, and GON induced with SP14 and SPA7 protocols. aPVAT adipocytes induced by SP14 and SPA and SPA induced GON adipocytes secreted more adiponectin in the medium compared to GON SP14, and mPVAT induced by SPA and SP14 (Fig. 5B). The lowest secretion of adiponectin was observed in SP14 induced mPVAT adipocytes (Fig. 5B).
Fig. 5.
Coculture with primary adipocytes enhances the lipolytic response to isoproterenol and adiponectin secretion in stromal vascular fraction (SVF) derived adipocytes compared to those induced with standard protocol. Preadipocytes from aortic (aPVAT) and mesenteric (mPVAT) perivascular adipose tissue and perigonadal (GON) adipose depots were differentiated by coculture with primary adipocytes for 7 days (SPA7) or standard protocol for 7 (SP7) and 14 days (SP14). A Lipolysis was stimulated with isoproterenol and released glycerol was quantified. Values are mean fold change over basal glycerol release ± SEM and adjusted to cell number (n = 5). B Adiponecitn release was quantified in the medium at the end of the induction period using an ELISA kit. Values are ng of adiponectin per mL per ng of protein. Significant differences are indicated by letters a, b, and c (P < 0.05)
Discussion
In this study, we demonstrate that adipogenesis induction of SVF-derived preadipocytes from PVAT by coculture with primary adipocytes (SPA7) reduced the culture time to complete induction and enhanced adipocyte functions including lipid accumulation and lipolytic responses to beta-adrenergic agonists. Adipocytes obtained by this method are phenotypically and functionally similar to primary adipocytes from the same depot. This coculture method has been previously used in the differentiation of subcutaneous white preadipocytes (Stacey et al. 2009), but not before tested in the induction of adipogenesis in preadipocytes from PVAT.
Coculture protocols as SPA7 take advantage of the paracrine signaling that regulates adipocyte differentiation. Mature adipocytes secrete growth factors, cytokines, and fatty acids that are inducers of adipogenesis (Zhao et al. 2015). Recently characterized pro-adipogenic growth factors include epidermal growth factor, leukemia inhibitory factor, platelet-derived growth factor BB, and basic fibroblast growth factor (Macotela et al. 2012). Cytokines such as CXCL3 (Kusuyama et al. 2016) and bone morphogenic proteins 2 and 4 (Macotela et al. 2012) are potent inducers of adipocyte maturation. Among fatty acids, oleate and linoleate induce adipocyte differentiation (Kokta et al. 2008). However, adipocytes’ secretome appears to be adipose depot specific (Roca-Rivada et al. 2011), and it changes during diseases such as diabetes, obesity (Knebel et al. 2017), and cardiovascular diseases (Fuster et al. 2016). Therefore, protocols like SPA7 could be advantageous in reductionist studies of PVAT responses during disease as the adipogenic induction of SVF-derived preadipocytes would be performed in an environment conditioned by primary adipocytes from the diseased animal.
Optimization of SPA times demonstrated that primary adipocytes retained their viability up to 144 h post collection. Classic studies showed that adipocytes remain viable for up to 9 days in culture and that their capacity to respond to insulin stimulus is not altered after 6 days in culture (Fried and Moustaid-Moussa 2001; Marshall et al. 1984). The capacity of primary adipocytes to retain their viability for longer periods of time supports the use of the coculture system in studies where having a large set of cultured adipocytes from the same animal would be advantageous. It is important to note that the populations of primary adipocytes used in this study were not characterized histologically or by flow cytometry and may contain immune cells such as macrophages. This is an important factor to consider when using coculture approaches, such as SPA7, in obese animals since macrophages tightly adhere to adipocytes and many will remain in the buoyant cell (adipocyte) fraction after collagenase digestion (Ebke et al. 2014).
SVF-derived preadipocytes exhibit differences in their adipogenic potential depending on their anatomical depot of origin. Visceral preadipocytes such as those from mPVAT and GON have lower adipogenic potential and their functional activity post-differentiation is lower than subcutaneous adipocytes (Baglioni et al. 2012). Reflecting this characteristic of visceral adipocytes, preadipocytes induced by SPA7 and SP14 exhibited lower expression of major adipogenic markers Adipoq, FABP4, and Plin1 compared to whole tissue samples (WAT). However, SPA7 protocol was more efficient in enhancing the transcription of these adipogenic genes compared to SP14. This advantage of SPA7 protocol is important as limited yields of differentiated PVAT adipocytes per number of preadipocytes seeded is a common limitation of standard pharmacological protocols of adipogenic induction.
The gene expression analysis of adipocytes induced by SPA7 demonstrated that these cells maintained their site-specific phenotypic signatures after 7 days of adipogenic stimuli. Recently Tran et al. (2018) established that adipocyte progenitors obtained by culturing thoracic aorta fragments under adipogenic conditions had a higher expression of brown phenotype-specific gene markers Dio2 and Ucp1 upon exposure to adipogenic medium for 13 days compared to adipocyte progenitors from the abdominal aorta. Similarly, in our study, the gene expression of Prdm16 and Ucp1 was significantly higher in aPVAT compared to mPVAT, while Dio2 was only detected in aPVAT. Thus, the SPA7 protocol successfully provides cultured adipocytes from SVF-derived preadipocytes that faithfully maintain the depot-specific PVAT phenotype.
The capacity to secrete adiponectin is an important feature of the terminal differentiation of adipocytes (Moseti et al. 2016). Although adiponectin is a canonical adipocyte protein, there are differences in the secretion levels depending on the site (Lihn et al. 2004). Using a classic pharmacological induction protocol Chatterjee et al. (2009) reported a lower expression of adiponectin in coronary artery PVAT differentiated adipocytes compared to that of cells from perirenal and subcutaneous depots. Secretion of adiponectin in cultured aPVAT adipocytes was reported by Vargas et al. (2017) but without comparing to abdominal PVAT. In the present study, we show that SPA increased adiponectin secretion in all depots tested. However, the differences in adiponectin secretion were maintained with mPVAT secreting less adiponectin compared to aPVAT, a difference reported in experiments comparing abdominal and thoracic PVAT (Tran et al. 2018). By enhancing secretion of adiponectin, SPA differentiated adipocytes dramatically increased their lipid accumulation compared to SP. An adipogenic model that supports adequate secretion of adiponectin is important in studies of PVAT, given the importance of this adipokine in vascular modulation.
Conclusion
Our results demonstrate that the adipogenesis efficiency in PVAT preadipocytes is enhanced by coculture with site-specific primary adipocytes from aPVAT and mPVAT. This is evident by the lipid accumulation in cells that were differentiated by SPA compared to those induced with SP. The use of a coculture system to induce adipogenesis in SVF-derived preadipocytes is a practical approach that reduces culture time, minimize the use of resources while producing adipocytes with a phenotype that faithfully reflects their anatomical location origin.
Acknowledgements
This research was supported by NHLBI 5R01HL117847-02 and 2P01HL070687-11A1. The authors acknowledge the technical assistance of Drs. Rahul Nelli and Clarissa Strieder-Barboza at the Department of Large Animal Clinical Sciences and Janice Thompson at the Watts Laboratory, Department of Pharmacology and Toxicology.
Animal rights statement
All animal procedures were approved by the Michigan State University Animal Care and Use Committee Procedures.
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A, Borgogni E, Frontera S, Nesi G, Liotta F, Lucchese M, Perigli G, Francini F, Forti G, Serio M, Luconi M. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS ONE. 2012;7:e36569. doi: 10.1371/journal.pone.0036569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzoska M, Geiger H, Gauer S, Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005;330:142–150. doi: 10.1016/j.bbrc.2005.02.141. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras GA, Thelen K, Ayala-Lopez N, Watts SW. The distribution and adipogenic potential of perivascular adipose tissue adipocyte progenitors is dependent on sexual dimorphism and vessel location. Physiol Rep. 2016;4:e12993. doi: 10.14814/phy2.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras GA, Strieder-Barboza C, de Souza J, Gandy J, Mavangira V, Lock AL, Sordillo LM. Periparturient lipolysis and oxylipid biosynthesis in bovine adipose tissues. PLoS ONE. 2017;12:e0188621. doi: 10.1371/journal.pone.0188621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebke LA, Nestor-Kalinoski AL, Slotterbeck BD, Al-Dieri AG, Ghosh-Lester S, Russo L, Najjar SM, von Grafenstein H, McInerney MF. Tight association between macrophages and adipocytes in obesity: implications for adipocyte preparation. Obesity (Silver Spring, Md.) 2014;22:1246–1255. doi: 10.1002/oby.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SK, Moustaid-Moussa N. Culture of Adipose Tissue and Isolated Adipocytes. In: Ailhaud G, editor. Adipose tissue protocols. New York: Springer; 2001. pp. 197–212. [DOI] [PubMed] [Google Scholar]
- Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118:1786–1807. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A, Ayala-Lopez N, Ahmad M, Watts SW. 3T3-L1 cells and perivascular adipocytes are not equivalent in amine transporter expression. FEBS Lett. 2017;591:137–144. doi: 10.1002/1873-3468.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebel B, Goeddeke S, Poschmann G, Markgraf DF, Jacob S, Nitzgen U, Passlack W, Preuss C, Dicken HD, Stuhler K, Hartwig S, Lehr S, Kotzka J. Novel insights into the adipokinome of obese and obese/diabetic mouse models. Int J Mol Sci. 2017;18:E1928. doi: 10.3390/ijms18091928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokta TA, Strat AL, Papasani MR, Szasz JI, Dodson MV, Hill RA. Regulation of lipid accumulation in 3T3-L1 cells: insulin-independent and combined effects of fatty acids and insulin. Animal Int J Animal Biosci. 2008;2:92–99. doi: 10.1017/S1751731107000936. [DOI] [PubMed] [Google Scholar]
- Kusuyama J, Komorizono A, Bandow K, Ohnishi T, Matsuguchi T. CXCL3 positively regulates adipogenic differentiation. J Lipid Res. 2016;57:1806–1820. doi: 10.1194/jlr.M067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Gollasch M. A clinical perspective: contribution of dysfunctional perivascular adipose tissue (PVAT) to cardiovascular risk. Curr Hypertens Rep. 2016;18:82. doi: 10.1007/s11906-016-0692-z. [DOI] [PubMed] [Google Scholar]
- Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol. 2004;219:9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S, Garvey WT, Geller M. Primary culture of isolated adipocytes. A new model to study insulin receptor regulation and insulin action. J Biol Chem. 1984;259:6376–6384. [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem cells (Dayton, Ohio). 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Morimoto C, Kameda K, Tsujita T, Okuda H. Relationships between lipolysis induced by various lipolytic agents and hormone-sensitive lipase in rat fat cells. J Lipid Res. 2001;42:120–127. [PubMed] [Google Scholar]
- Moseti D, Regassa A, Kim WK. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci. 2016;17(pii):E124. doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul, Integr Comp Physiol. 2013;304:R543–R552. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H460–H465. doi: 10.1152/ajpheart.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Rivada A, Alonso J, Al-Massadi O, Castelao C, Peinado JR, Seoane LM, Casanueva FF, Pardo M. Secretome analysis of rat adipose tissues shows location-specific roles for each depot type. J Proteom. 2011;74:1068–1079. doi: 10.1016/j.jprot.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ojeda FJ, Rupérez AI, Gomez-Llorente C, Gil A, Aguilera CM. Cell models and their application for studying adipogenic differentiation in relation to obesity: a review. Int J Mol Sci. 2016;17(pii):E1040. doi: 10.3390/ijms17071040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hung CM, Guertin DA. Emerging complexities in adipocyte origins and identity. Trends Cell Biol. 2016;26:313–326. doi: 10.1016/j.tcb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham N, Gefen A. Stochastic modeling of adipogenesis in 3T3-L1 cultures to determine probabilities of events in the cell’s life cycle. Ann Biomed Eng. 2011;39:2637–2653. doi: 10.1007/s10439-011-0341-2. [DOI] [PubMed] [Google Scholar]
- Stacey DH, Hanson SE, Lahvis G, Gutowski KA, Masters KS. In vitro adipogenic differentiation of preadipocytes varies with differentiation stimulus, culture dimensionality, and scaffold composition. Tissue Eng Part A. 2009;15:3389–3399. doi: 10.1089/ten.tea.2008.0293. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- Thelen K, Ayala-Lopez N, Watts SW, Contreras GA. Expansion and adipogenesis induction of adipocyte progenitors from perivascular adipose tissue isolated by magnetic activated cell sorting. J Vis Exp. 2017 doi: 10.3791/55818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KV, Fitzgibbons T, Min SY, DeSouza T, Corvera S. Distinct adipocyte progenitor cells are associated with regional phenotypes of perivascular aortic fat in mice. Mol Metab. 2018;9:199–206. doi: 10.1016/j.molmet.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas D, Camacho J, Duque J, Carreno M, Acero E, Perez M, Ramirez S, Umana J, Obando C, Guerrero A, Sandoval N, Rodriguez G, Lizcano F. Functional characterization of preadipocytes derived from human periaortic adipose tissue. Int J Endocrinol. 2017;2017:2945012. doi: 10.1155/2017/2945012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R, Fink GD. Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol. 2013;33:1320–1328. doi: 10.1161/ATVBAHA.113.301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Chiba S, Moriwaki C, Kitamura H, Ina K, Aosa T, Tomonari K, Gotoh K, Masaki T, Katsuragi I, Noguchi H, Kakuma T, Hamaguchi K, Shimada T, Fujikura Y, Shibata H. A clinical approach to brown adipose tissue in the para-aortic area of the human thorax. PLoS ONE. 2015;10:e0122594. doi: 10.1371/journal.pone.0122594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Waldman SD, Flynn LE. Multilineage co-culture of adipose-derived stem cells for tissue engineering. J Tissue Eng Regen Med. 2015;9:826–837. doi: 10.1002/term.1643. [DOI] [PubMed] [Google Scholar]