Abstract
Glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), produced by intestinal enteroendocrine L cells, are important gut hormones that coordinate gastrointestinal physiology, metabolism, and appetite. We aimed to investigate the role of olfactory receptor (OR) OR51E1 in GLP-1 and PYY secretion. We analyzed the expression of olfactory marker protein (OMP), an indicator of OR-mediated events in nonolfactory systems, in human intestinal L cells. Furthermore, we analyzed OMP and OR51E1 expression in the L cell line NCI-H716. To investigate whether odorant-activated OR signaling stimulates GLP-1 and PYY secretion, we used nonanoic acid, a known OR51E1 ligand. Treatment with 100 μM nonanoic acid increased GLP-1 secretion by 2.32 ± 0.41-fold and PYY secretion by 1.44 ± 0.10-fold; however, this effect was attenuated on small interfering RNA-mediated OR51E1 knockdown. Oral administration of nonanoic acid to rats resulted in a 2.89 ± 0.53-fold increase in GLP-1 levels and reductions in blood glucose levels compared with the control group. Nonanoic acid stimulates GLP-1 and PYY secretion via OR51E1 signaling in L cells, thereby indicating a potential role of OR-mediated events in GLP-1 and PYY secretion; this could be translated into a therapeutic approach in treating diabetes.
Keywords: ectopic OR expression, enteroendocrine L cells, GLP-1, nonanoic acid, OR51E1
Glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) are produced and secreted from intestinal enteroendocrine L cells, their plasma levels rapidly increase within minutes of food intake [1–3], and their corelease is known to suppress appetite acutely [4]. Secreted GLP-1 has insulinotropic activity; its upregulation is considered a potential pharmacological target in treating type 2 diabetes, and GLP-1 receptor agonists, e.g., liraglutide, are currently in use for the treatment of type 2 diabetes [5, 6]. Recently, expression of olfactory receptors (ORs), which belong to the G-protein-coupled receptors, and occurrence of OR-associated events were reported in nonolfactory tissues, primarily in those sensing extracellular signals [7]. In the olfactory system, the binding of specific odorants to ORs on OR neurons initiates a signal transduction cascade involving olfactory-specific GTP protein, adenylyl cyclase 3, and the olfactory marker protein (OMP) [8]. OMP is involved in Ca2+ extrusion during olfactory signal transduction, and its expression is considered an indicator of OR-mediated chemoreception in nonolfactory systems [9]. Although ORs in endocrine tissues, including the pancreas and thyroid, are involved in hormone secretion, physiological functions of ORs in many other nonolfactory tissues in which they have been detected remain unknown [7, 10].
The current study aimed to investigate the role of the OR OR51E1 in GLP-1 and PYY secretion. We confirmed nonolfactory OMP expression in human enteroendocrine L cells and hypothesized that odorants stimulate GLP-1 and PYY secretion through OR activation. Among ORs expressed in the NCI-H716 cell line, we focused on the expression and role of OR51E1 because of its reported expression and colocalization in enteroendocrine cells in vivo [11]. Furthermore, we investigated the in vivo effects of odorant-stimulated GLP-1 secretion in Sprague-Dawley rats.
1. Materials and Methods
A. Cell Culture
NCI-H716, a human L cell line derived from cecal adenocarcinoma, was purchased from Korean Cell Line Bank (Seoul, Korea). Cells were cultured in RPMI 1640 medium (Hyclone, Logan, UT) containing 10% fetal bovine serum and 1% penicillin/streptomycin. DMEM containing 10% fetal bovine serum and 1% penicillin/streptomycin was used for differentiation, and Dulbeccos PBS was supplemented with Ca2+ and Mg2+ for experiments.
B. In Vitro Analysis
Cells were seeded on Matrigel-coated plates and allowed to differentiate for 48 hours. After stimulant treatment, Western blot analysis was performed, as previously described [12], using anti-phosphorylated (p)-ERK (1:2000; Cat. #4370; Cell Signaling Technology Danvers, MA; RRID:AB_2315112[13]), anti-total (t)-ERK (1:2000; Cat. #9102; Cell Signaling Technology; RRID:AB_330744[14]), and anti-β-actin (Santa Cruz Biotechnology Dallas, TX; RRID:AB_2714189[15]) antibodies. For GLP-1 secretion, cells were treated with stimulants along with a 50-μM dipeptidyl peptidase-4 inhibitor (MilliporeSigma, Burlington, MA) for 2 hours after 48 hours of differentiation. Media were analyzed using an active GLP-1 ELISA kit (EGLP-35K; Thermo Fisher Scientific, Waltham, MA) and PYY ELISA kit (EZHPYYT66K; MilliporeSigma). cAMP was assessed using cAMP XP® Assay Kit (Cell Signaling Technology), according to the manufacturer’s instructions.
C. siRNA Transfection and PCR Analysis
Small interfering RNA (siRNA) targeting OR51E1 (HSS135044; Thermo Fisher Scientific) was transfected into the cells using Lipofectamine RNAiMAX (Thermo Fisher Scientific). siRNA was transfected into the cells as a suspension for 6 hours and then seeded onto the Matrigel-coated well and differentiated for 48 hours before the experiment. PCR was performed using C1000TM Thermal Cycler (Bio-Rad Laboratories, Hercules, CA) with HelixAmpTM Ready-2x-Go Taq (NanoHelix, Daejeon, Korea). The OR51E1 primer set used for PCR was as follows: forward primer, 5′-TCCTCATCTCCACCTCATCCA-3′; reverse primer, 5′-CACAGCAGCCACACCAATTTT-3′. The cycle conditions were as follows: 95°C for 2 minutes, followed by 33 cycles at 95°C for 20 seconds, 60°C for 40 seconds, and 72°C for 1 minute, and finally, 72°C for 5 minutes.
D. Immunofluorescence Staining
Paraffin-embedded, 4 μm-thick human ileum sections were stained, as previously described [16], using anti-OMP (1:500; sc-67219; Santa Cruz Biotechnology), anti-GLP-1 (1:500; sc-7782; Santa Cruz Biotechnology), and anti-OR51E1 (1:500; LS-A1852; LifeSpan BioSciences, Seattle, WA) antibodies. NCI-H716 cells were fixed with 4% paraformaldehyde, permeabilized with 1% Triton X-100, and blocked with 10% normal donkey serum before incubation with primary antibodies overnight at 4°C. Sections or cells were incubated with fluorescence-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA), mounted with Vectashield medium (Vector Laboratories, Burlingame, CA), and visualized under an LSM 700 laser-scanning confocal microscope (Carl Zeiss, Oberkochen, Germany).
E. In Vivo Analysis
Eight-week-old Sprague-Dawley rats were obtained from Japan SLC, Inc. (Hamamatsu, Japan), and all experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Yonsei University Severance Hospital (Seoul, Korea; Approval No. 2015-0025). Rats were unfed for 16 hours before an oral glucose tolerance test (OGTT) with 2 g/kg glucose, and blood samples were collected through retro-orbital bleeding under anesthesia induced via isoflurane inhalation (Hana Pharm, Seoul, Korea). Dipeptidyl peptidase-4 inhibitor was added to the blood samples and analyzed using a total GLP-1 ELISA kit (EZGLP1T-36K; MilliporeSigma). Blood glucose levels were determined with a glucometer (Arkray, Minneapolis, MN) using tail nick bleeding.
F. Human Samples
A pathologically disease-free proximal resection margin of the ileum was obtained from a colon cancer patient undergoing a right hemicolectomy. Clinicopathological parameters, such as local invasion and distant metastasis, were retrospectively collected. The study was approved by the Institutional Review Board of Yonsei University (Institutional Review Board Number: 4-2017-1157).
G. Statistical Analysis
ImageJ software was used for densitometric analysis of the Western blots. Calculations and statistical analysis were carried out using GraphPad Prism 6 software. All experiments were performed in triplicate, and data were analyzed using Student t tests and presented as means ± SD. P < 0.05 was considered significant.
2. Results
A. OMP and OR Expression in Enteroendocrine L Cells
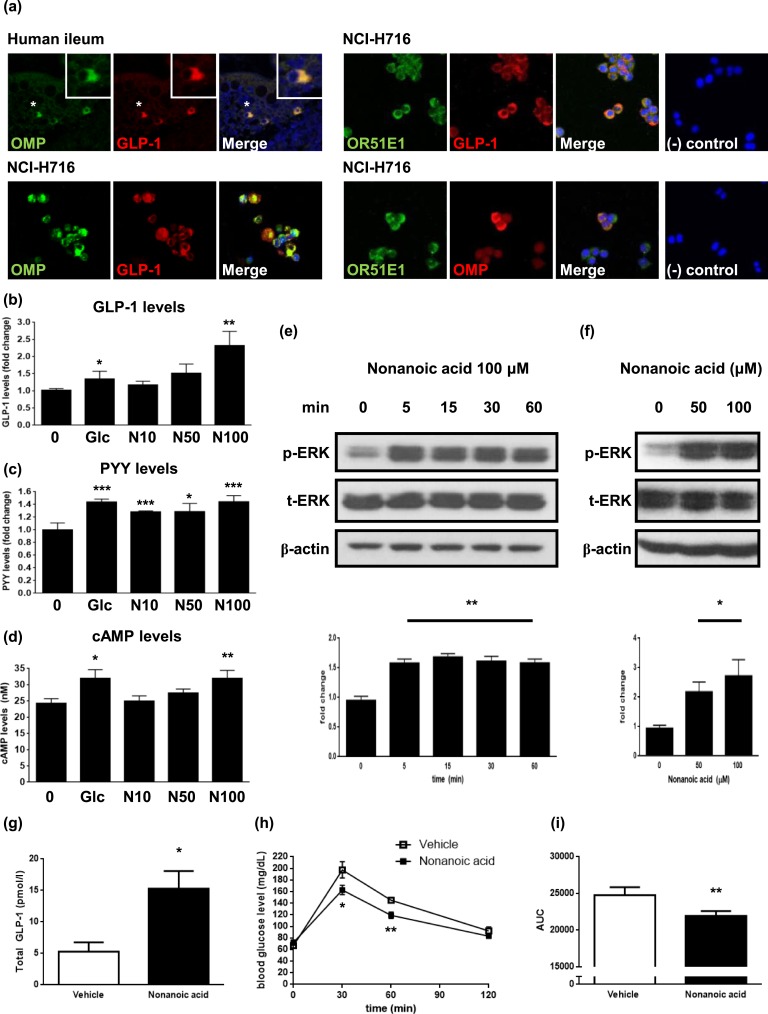
The ectopic expression of OMP is considered an indicator of potential OR-mediated events in nonolfactory systems [7]. As the OR Olfr544 is involved in azelaic acid-stimulated glucagon secretion in pancreatic enteroendocrine α cells [10], we hypothesized that OR-associated events occur within intestinal enteroendocrine cells, specifically GLP-1-secreting L cells. Immunofluorescence staining revealed OMP expression in human intestinal L cells and colocalization of OMP, OR51E1, and GLP-1 in NCI-H716 cells, which serves as an in vitro model for enteroendocrine L cells [Fig. 1(a)].
Figure 1.
Nonanoic acid stimulates GLP-1 secretion in OR51E1-expressing enteroendocrine L cells. (a) Expression of OMP in GLP-1-secreting L cells in the human ileum section (upper, left) and NCI-H716 cells (lower, left). Boxed areas represent higher magnification of selected cells (indicated with asterisks). Representative images showing colocalization of OMP, OR51E1, and GLP-1 in NCI-H716 cells (right). Negative controls showed no immunostaining in NCI-H716 cells. Images of human ileum were obtained with a laser-scanning microscope at magnification of ×200 and NCH-H716 cells at magnification of ×400. (b) NCI-H716 cells were incubated with different concentrations of nonanoic acid or 50 mM glucose for 2 h, and media were collected for analysis of secreted GLP-1 levels. The data in the column are obtained from mean fold change value ± SD of six independent experiments. (c) NCI-H716 cells were incubated with different concentrations of nonanoic acid or 50 mM glucose for 2 h, and media were collected for analysis of secreted PYY levels. The data in the column are obtained from mean fold change value ± SD of three independent experiments. (d) NCI-H716 cells were incubated with different concentrations of nonanoic acid or 50 mM glucose for 30 min, and intracellular cAMP levels were assessed. (e) NCI-H716 cells were treated with 100 µM of nonanoic acid for indicated periods of time. Bands are representative of three independent experiments, and data in the column are obtained from mean relative density ratios ± SD of normalized values by densitometry. (f) NCI-H716 cells were treated with different concentrations of nonanoic acid for 15 min. Bands are representative of three independent experiments, and data in the column are obtained from mean relative density ratios ± SD of normalized values by densitometry. (g) Nonanoic acid (500 mg/kg; n = 5) or vehicle (saline; n = 4) was orally administered to rats, and blood samples were drawn 15 min after oral administration and analyzed for total GLP-1 concentrations. (h) Nonanoic acid (500 mg/kg; ▪) or vehicle (□) were orally administered to rats (n = 8 per each group), 30 min before OGTT. Plasma glucose levels were measured at 0 (baseline), 30, 60, and 120 min after glucose administration. (i) AUC was calculated from time 0 to 120 min of OGTT graph. (b)–(f) *P < 0.05, **P < 0.01, ***P < 0.001 vs control; (g)–(i) *P < 0.05, **P < 0.001 vs vehicle. 0, control; Glc, glucose 50 mM; N10, N50, and N100, 10 μM , 50 μM, and 100 μM nonanoic acid, respectively. AUC, area under the curve.
B. Nonanoic Acid Stimulates GLP-1 and PYY Secretion in NCI-H716 Cells
To investigate whether OR51E1 exerts a physiological function in NCI-H716, we treated these cells with nonanoic acid, a known OR51E1 ligand. Butyl butyryl lactate is also a known ligand for OR51E1, but we focused on nonanoic acid, which is a C9:0 fatty acid with the formula of CH3(CH2)7COOH [17]. Cells were incubated with different concentrations of nonanoic acid or 50 mM glucose (positive control) for 2 hours, and GLP-1 and PYY secretion was assessed. Nonanoic acid dose dependently increased GLP-1 and PYY secretion, and at 100 μM, secretion was increased by a factor of 2.32 ± 0.41 (P < 0.01) compared with dimethyl sulfoxide-treated control for GLP-1 [Fig. 1(b)] and 1.44 ± 0.10 (P < 0.001) for PYY [Fig. 1(c)]. To evaluate whether a high-glucose concentration affects nonanoic acid-stimulated GLP-1 secretion, we performed a secretion experiment in the presence of 50 mM glucose. Although nonanoic acid still stimulated GLP-1 secretion dose dependently, it did not have any additive effect with the glucose (data not shown). As stimulation of GLP-1 and PYY secretion by fatty acids involves cAMP production and p-ERK [4, 15–18], cAMP levels were elevated in a dose-dependent manner [P < 0.01 at 100 μM; Fig. 1(d)], and ERK was activated by 100 μM nonanoic acid [P < 0.01; Fig. 1(e)]. Assessment of the effect of nonanoic acid at different concentrations revealed that the degree of activation is also dose dependent [P < 0.05; Fig. 1(f)].
C. Oral Administration of Nonanoic Acid Increases Plasma GLP-1 Levels in Rats
We next assessed the in vivo effects of nonanoic acid. We administered 500 mg/kg of nonanoic acid (n = 5) or vehicle (saline; n = 4) via oral gavage to Sprague-Dawley rats, and blood samples were drawn 15 minutes after administration. The mean level of total circulating GLP-1 was 5.29 ± 0.73 pmol/L in the control group and 15.27 ± 2.82 pmol/L in the nonanoic acid group [P < 0.05; Fig. 1(g)]. We also performed an OGTT, 30 minutes after administration. Nonanoic acid-treated rats had significantly lower blood glucose levels than the control group with P < 0.05 at 30 minutes and P < 0.01 at 60 minutes [Fig. 1(h)]. This translated into ~11% lower glucose area-under-the-curve values for 120 minutes after OGTT [P < 0.01; Fig. 1(i)].
D. OR51E1 Knockdown Reduces Nonanoic Acid-Stimulated GLP-1 and PYY Secretion in NCI-H716 Cells
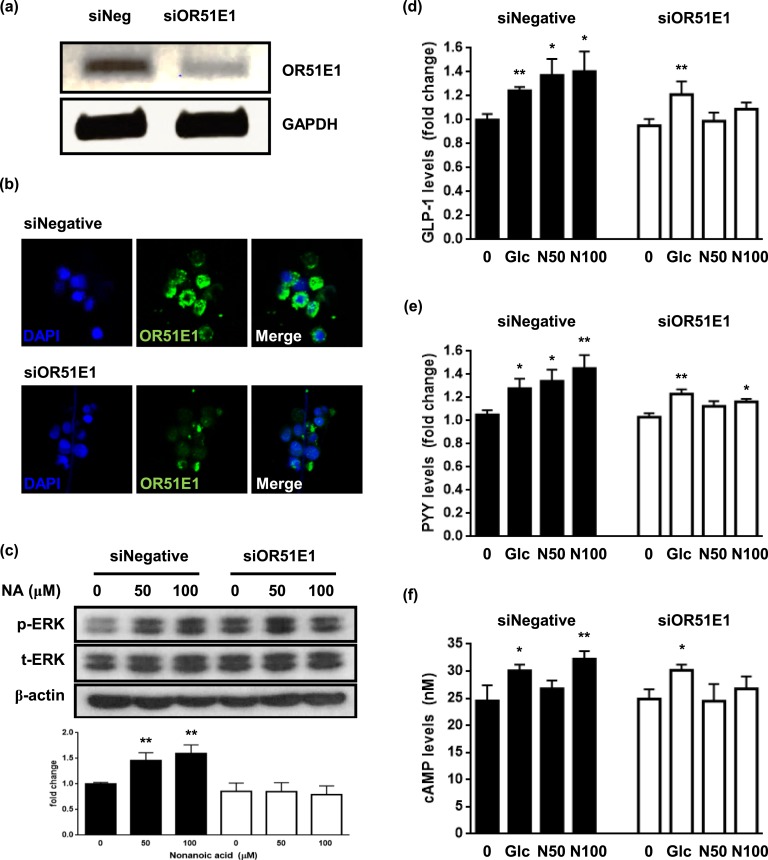
To confirm the involvement of OR51E1 in nonanoic acid-stimulated GLP-1 secretion, we treated NCI-H716 cells with siRNA targeting human OR51E1 mRNA. Knockdown was successful; i.e., OR51E1 mRNA levels [P < 0.01; Fig. 2(a)], as well as immunofluorescence signal [Fig. 2(b)], were reduced and resulted in attenuation of GLP-1 secretion [Fig. 2(d)], PYY secretion [Fig. 2(e)], p-ERK [Fig. 2(c)], and cAMP production [Fig. 2(f)]. This indicates that GLP-1 and PYY stimulation by nonanoic acid is regulated by the OR51E1-mediated signaling pathway.
Figure 2.
OR51E1 knockdown attenuates nonanoic acid-stimulated GLP-1 secretion in NCI-H716 cells. (a) mRNA levels of OR51E1 after transfection with OR51E1-specific siRNA (siNegative, siOR51E1) were analyzed using PCR analysis. (b) Cells were fixed 48 h after the transfection with OR51E1-specific siRNA, and immunofluorescence staining was performed. Images were obtained with a laser-scanning microscope at magnification of ×800. (c) NCI-H716 cells were treated with indicated concentrations of nonanoic acid for 15 min. Bands are representative of three independent experiments. p-ERK was quantified by densitometry and normalized to the t-ERK level for each sample. The data in the column are obtained from mean relative density ratios ± SD of three repeated experiments. (d) Transfection with OR51E1-specific siRNA reduced nonanoic acid-induced GLP-1 secretion in NCI-H716 cells. (e) Transfection with OR51E1-specific siRNA reduced nonanoic acid-induced PYY secretion in NCI-H716 cells. (f) Transfection with OR51E1-specific siRNA reduced nonanoic acid-induced cAMP production. *P < 0.05, **P < 0.01 vs control. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NA, nonanoic acid.
3. Discussion
As the roles of ORs in OMP-expressing nonolfactory tissues have received increasing attention [7, 19, 20], we analyzed OMP and OR51E1 expression in the human enteroendocrine L cell line NCI-H716. Together with other enteroendocrine cells, such as enterochromaffin cells and K cells, L cells sense luminal content and secrete hormones that control gut motility, insulin secretion, and gastric emptying [21–23]. Moreover, enteroendocrine cells may sense odorants in the luminal environment of the gut via an OR-mediated pathway [22, 24]. Therefore, we hypothesized that luminal odorants bind ORs expressed on L cells and stimulate GLP-1 secretion.
Nonanoic acid, a known OR51E1 ligand, is a fatty acid [17], and along with glucose, fatty acids are potent physiological regulators of GLP-1 and PYY, acting via receptors, including G-protein-coupled receptors 120 and 84 (Gpr120 and Gpr84) [18, 25, 26]. Fatty acids stimulate GLP-1 from STC-1 cells via the Gpr120 receptor [18]; however, the Gpr120 receptor is not expressed in NCI-H716 cells. Moreover, the effects of nonanoic acid or OR-mediated signaling pathways on GLP-1 and PYY secretion are yet unclear.
Here, treatment of NCI-H716 cells with nonanoic acid increased GLP-1 and PYY secretion, along with cAMP production and p-ERK. On OR51E1 knockdown, nonanoic acid-stimulated GLP-1 and PYY secretion and related responses were reduced, indicating that they are regulated via OR51E1 signaling. Oral administration of nonanoic acid to rats increased circulating GLP-1 levels. Based on our in vitro results, we assume that this effect may be mediated by olr63, the rat ortholog of OR51E1.
Although further studies with larger samples and diabetes mellitus mouse models are required, our finding that nonanoic acid enhances GLP-1 and PYY secretion indicates that the specific odorants or their derivatives may be developed as potential antidiabetic treatment agents. The implication of OR-mediated events in GLP-1 and PYY secretion suggests that the corresponding pathways could represent targets for enhancing GLP-1 and PYY levels in patients with type 2 diabetes.
Acknowledgments
We acknowledge Ki-Suk Kim from Kyung Hee University for providing helpful suggestions on experimental techniques.
Financial Support: This study was supported by the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs (No. HI13C0423).
Author Contributions: Y.E.H., C.W.K., and J.H.O. carried out the experiments. Y.E.H. wrote the manuscript with help from C.R.K., Y.H.C., M.K.L., and E.J.L. S.H.P. and E.J.L. conceived of the original idea, and E.J.L. supervised the project. All authors provided critical feedback and contributed to the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- GLP-1
glucagon-like peptide-1
- Gpr84
G-protein-coupled receptor 84
- Gpr120
G-protein-coupled receptor 120
- OGTT
oral glucose tolerance test
- OMP
olfactory marker protein
- OR
olfactory receptor
- p-ERK
phosphorylated ERK
- PYY
peptide YY
- siRNA
small interfering RNA
- t-ERK
total ERK
References and Notes
- 1. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. [DOI] [PubMed] [Google Scholar]
- 2. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. [DOI] [PubMed] [Google Scholar]
- 3. De Silva A, Bloom SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes. 2015;39(3):424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21. [DOI] [PubMed] [Google Scholar]
- 6. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Suppl 2):S279–S284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang N, Kim H, Jae Y, Lee N, Ku CR, Margolis F, Lee EJ, Bahk YY, Kim MS, Koo J. Olfactory marker protein expression is an indicator of olfactory receptor-associated events in non-olfactory tissues. PLoS One. 2015;10(1):e0116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dibattista M, Reisert J. The odorant receptor-dependent role of olfactory marker protein in olfactory receptor neurons. J Neurosci. 2016;36(10):2995–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwon HJ, Koo JH, Zufall F, Leinders-Zufall T, Margolis FL. Ca extrusion by NCX is compromised in olfactory sensory neurons of OMP mice. PLoS One. 2009;4(1):e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang N, Bahk YY, Lee N, Jae Y, Cho YH, Ku CR, Byun Y, Lee EJ, Kim MS, Koo J. Olfactory receptor Olfr544 responding to azelaic acid regulates glucagon secretion in α-cells of mouse pancreatic islets. Biochem Biophys Res Commun. 2015;460(3):616–621. [DOI] [PubMed] [Google Scholar]
- 11. Priori D, Colombo M, Clavenzani P, Jansman AJ, Lallès J-P, Trevisi P, Bosi P. The olfactory receptor OR51E1 is present along the gastrointestinal tract of pigs, co-localizes with enteroendocrine cells and is modulated by intestinal microbiota. PLoS One. 2015;10(6):e0129501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han YE, Hwang S, Kim J, Byun JW, Yoon JS, Lee EJ. Biguanides, metformin and phenformin, generate therapeutic effects via AMP-activated protein kinase/extracellular-regulated kinase pathways in an in vitro model of Graves’ orbitopathy. Thyroid. 2018;28(4):528–536. [DOI] [PubMed] [Google Scholar]
- 13.RRID:AB_2315112. http://antibodyregistry.org/search?q=RRID:%20AB_2315112.
- 14.RRID:AB_330744. http://antibodyregistry.org/search?q=AB_330744.
- 15.RRID:AB-2714189. http://antibodyregistry.org/search?q=AB_2714189.
- 16. Kang CW, Han YE, Kim J, Oh JH, Cho YH, Lee EJ. 4-Hydroxybenzaldehyde accelerates acute wound healing through activation of focal adhesion signalling in keratinocytes. Sci Rep. 2017;7(1):14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a mammalian receptor repertoire. Sci Signal. 2009;2(60):ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11(1):90–94. [DOI] [PubMed] [Google Scholar]
- 19. Kang N, Koo J. Olfactory receptors in non-chemosensory tissues. BMB Rep. 2012;45(11):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feldmesser E, Olender T, Khen M, Yanai I, Ophir R, Lancet D. Widespread ectopic expression of olfactory receptor genes. BMC Genomics. 2006;7(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92(4):219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132(5):1890–1901. [DOI] [PubMed] [Google Scholar]
- 23. Beumer J, Clevers H. How the gut feels, smells, and talks. Cell. 2017;170(1):10–11. [DOI] [PubMed] [Google Scholar]
- 24. Fleischer J, Bumbalo R, Bautze V, Strotmann J, Breer H. Expression of odorant receptor Olfr78 in enteroendocrine cells of the colon. Cell Tissue Res. 2015;361(3):697–710. [DOI] [PubMed] [Google Scholar]
- 25. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Wu X, Simonavicius N, Tian H, Ling L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J Biol Chem. 2006;281(45):34457–34464. [DOI] [PubMed] [Google Scholar]