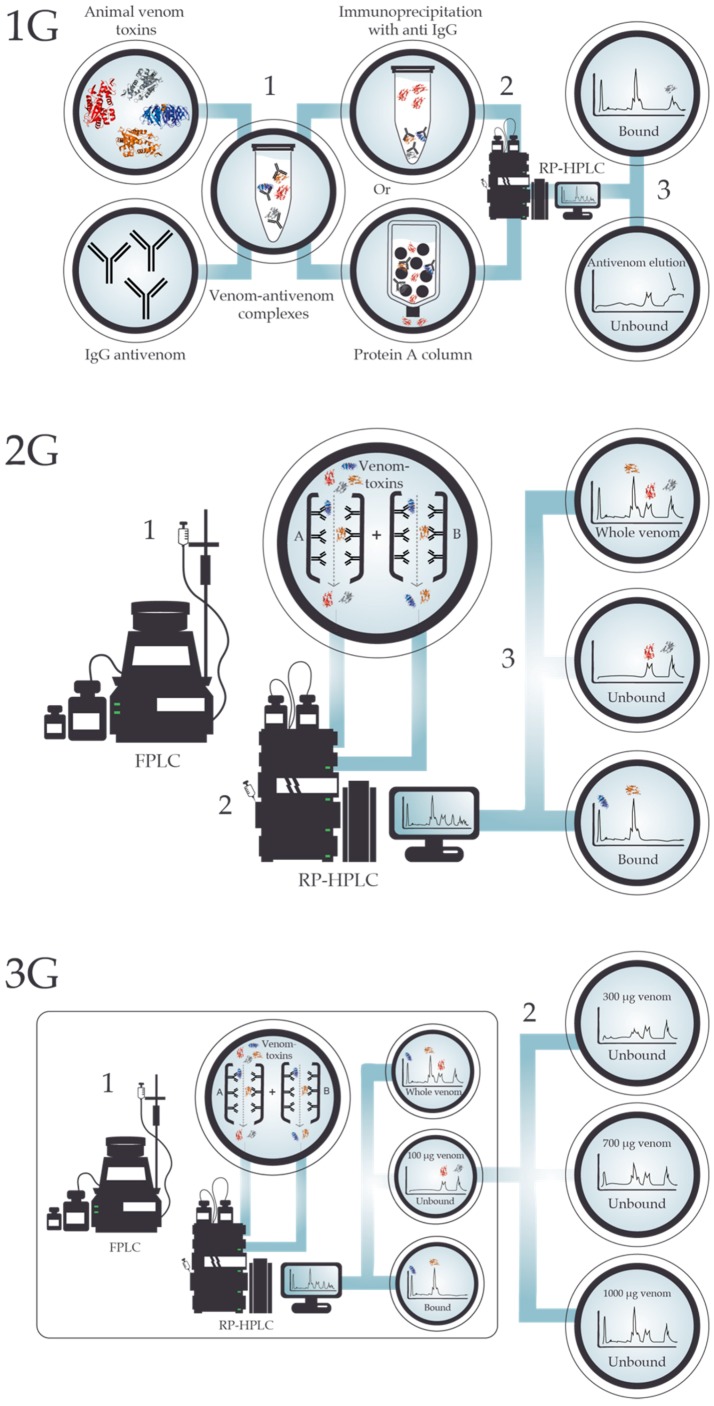
Figure 2.
The evolution of the antivenomics methodology. (1G) First generation antivenomics involves the formation of toxin-antibody complexes (1), which are then either immunoprecipitated or forced through a Protein A column to separate toxin-antibody complexes from unbound toxins (2). Both fractions are then analyzed by reversed-phase high performance liquid chromatography (RP-HPLC), which allows for the identification of bound and unbound toxins. (2G) In second generation antivenomics, antivenom molecules (whole antibodies or fragments) are covalently immobilized onto a chromatographic matrix (immunoaffinity column), and the venom of interest is forced through this column, with the unbound toxins running straight through (1). Thereafter, the bound and unbound toxins are analyzed by RP-HPLC (2). Finally, quantitative comparisons of RP-HPLC chromatograms of whole venom and the immunoaffinity column eluates can be made, thus providing qualitative and quantitative information on both the set of toxins bearing antivenom-recognized epitopes and those toxins exhibiting poor immunoreactivity (3). (3G) Third generation antivenomics builds upon the concept of the immunoaffinity column applied in 2G antivenomics and repeats the same initial procedure (1). However, a range of venom-antivenom concentrations are tested to determine the maximal binding capacity of an antivenom against each toxin in a venom, as well as the quantification of the fraction of toxin-specific antibodies present in the antivenom (2).