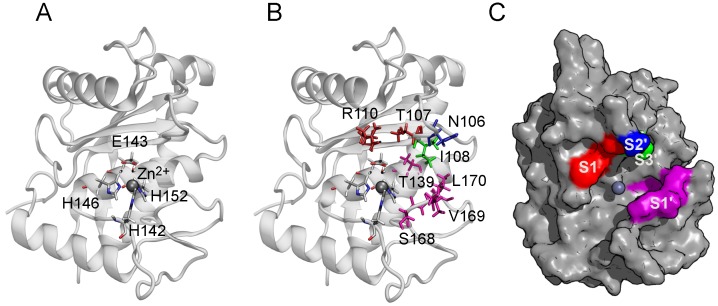
Figure 1.
Structure of BaP1 and location of the catalytic zinc and the substrate binding subsites. (A) The catalytic zinc ion is coordinated tetrahedrally by the N2 atoms of three histidine residues (His142, His146, His 152) and the oxygen atom of the catalytic water molecule (Wat67), coordinated by the residue Glu143. The Zn ion is shown as a gray sphere. The indicated amino acids are shown in a bond representation (H, white; C, gray; N, blue; O, red). (B) The side chains of amino acids belonging to the substrate binding subsites indicated by color: S1, red; S1, magenta; S2, blue; S3, green. (C) Surface of BaP1 showing the substrate binding subsites with the color code previously described. Coordinates of BaP1 were obtained from the Protein Data Bank (PDB code 2W15).