Fig. 1.
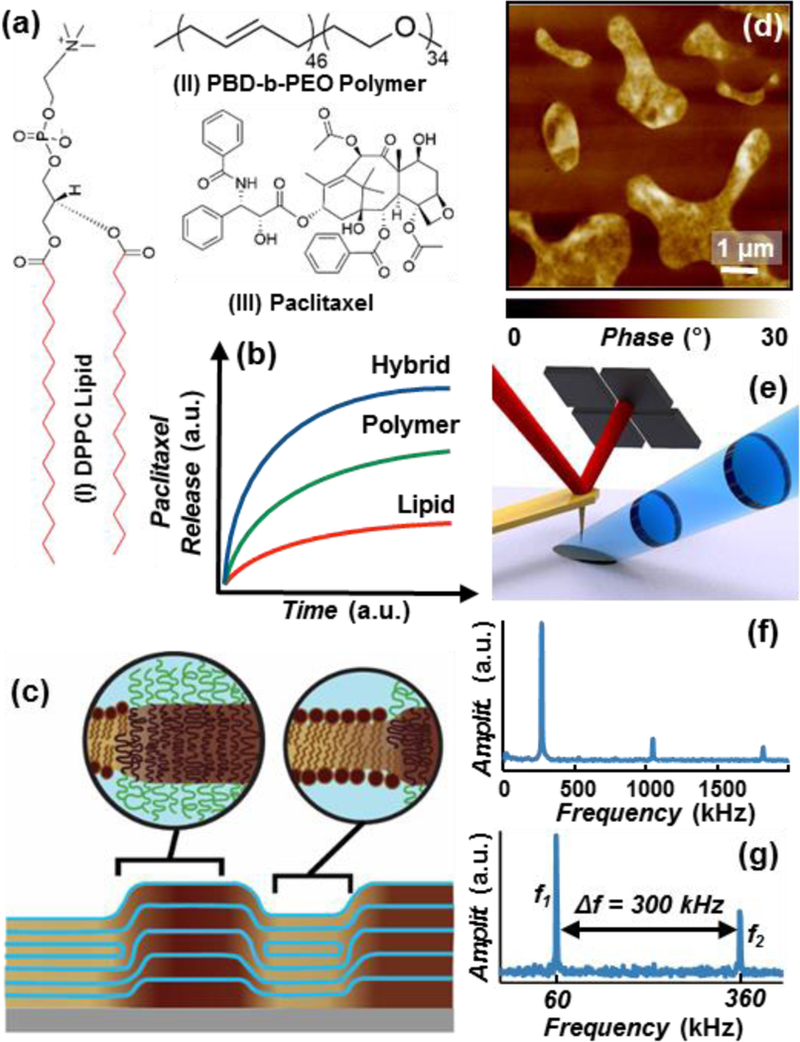
(a) Chemical structures of: (I) 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) lipid; the red bonds identify the deuterium substituted positions on the partially deuterated DPPC (d-DPPC), (II) poly(butadiene-b-ethylene oxide) block polymer (PBD-b-PEO) and (III) Paclitaxel. (b) Schematic of the paclitaxel cumulative release profiles from DPPC/PBD-b-PEO hybrid (blue), PBD-b-PEO (green), and DPPC (red) at parity initial paclitaxel load.15 (c) Schematic of the multi-layered DPPC/PBD-b-PEO hybrid films on solid substrate, depicting the self-assembled chemically-affine domains and domain boundaries perpendicular to the substrate. (d) Representative AFM phase image depicting drug-loaded DPPC/PBD-b-PEO hybrid films heterogeneity (phase separation). (e) PTIR Schematic: a pulsed tunable IR laser (blue) illuminates the portion of the sample centered around the gold-coated AFM probe. Light absorption in the sample induces local thermal expansion. The AFM cantilever transduces the expansion of the sample and provides nanoscale resolution. (f) In contact-mode PTIR experiments (i.e. spectra) the laser repetition rate matches the frequency of oscillation to the first cantilever mode (≈ 270 kHz). (g) In tapping-mode PTIR experiments (i.e. maps) the laser repetition rate matches the frequency difference (Δf) between the second (f2) and first (f1) modes of the cantilever.
