Abstract
Anti-tumor alkyl phospholipid analogs comprise a group of structurally related molecules with remarkable tumor selectivity. Some of these compounds have shown radiosensitizing capabilities. CLR127 is a novel, clinical-grade anti-tumor alkyl phospholipid ether analog, a subtype of synthetic alkyl phospholipids broadly targeting cancer cells with limited uptake in normal tissues. The purpose of this study was to investigate the effect of CLR127 to modulate radiation response across several adult and pediatric cancer types in vitro as well as in murine xenograft models of human prostate adenocarcinoma, neuroblastoma, Ewing sarcoma and rhabdomyosarcoma. In vitro, CLR127 demonstrated selective uptake in cancer cells compared to normal cells. In cancer cells, CLR127 treatment prior to radiation significantly decreased clonogenic survival in vitro, and led to increased radiation-induced dsDNA breakage compared to radiation alone, which was not observed in normal controls. In animal models, CLR127 effectively increased the antitumor response to fractionated radiation therapy and led to delayed tumor regrowth at potentially clinically achievable doses. In conclusion, our study highlights the ability of CLR127 to increase radiation response in several cancer types. Given almost universal uptake of CLR127 in malignant cells, future research should test whether the observed effects can be extended to other tumor types. Our data provide a strong rationale for clinical testing of CLR127 as a tumor-targeted radiosensitizing agent.
Keywords: Alkyl phospholipid, alkyl phospholipid ether, radiosensitization, tumor-targeted, DNA repair, radiotherapy
Introduction
Radiation plays a pivotal role in the treatment of most adult and pediatric solid tumors and has significantly contributed to the increase in overall cancer survival rates over the last several decades. However, the dose that can be safely delivered to tumors is often limited by acute and long-term adverse effects of radiation on surrounding normal tissues. Radiosensitizing drugs aim to increase tumor cell kill by radiation, ideally with little effect on normal tissues. The majority of clinically utilized radiosensitizers are DNA-targeting agents (such as platinum derivatives, gemcitabine or temozolomide), however their application can be limited by intrinsic toxicities and non-tumor selective modes of action, thereby inducing radiosensitization of normal tissues (1).
Anti-tumor alkyl phospholipids (APL) comprise a group of structurally related molecules which, in contrast to classical chemotherapeutic agents that act on DNA, target cellular and intracellular membranes (2). Some APL have demonstrated remarkable tumor selectivity, attributed in part to uptake via lipid rafts, which are much more abundant in cancer cell membranes compared to normal cells (3,4). The cytotoxic effects of APL on cancer cells are complex. APL, in contrast to classical chemotherapeutic agents, do not directly act on the DNA or DNA repair mechanisms, and do not enter the nucleus. Some of the known anti-cancer effects of this class of drugs include interference with phosphatidylcholine biosynthesis, inhibition of lipid-mediated signal transduction pathways and membrane microdomain formation (5). Some APL have been reported to have radiosensitizing capabilities, as they affect a variety of pathways involved in DNA damage repair, such as PI3K/Akt and SAPK/Jnk (4,6,7). Despite their tumor selectivity and anti-cancer properties, few APL have entered clinical trials and found to be efficacious, largely due to the lack of intravenous formulations, and dose-limiting gastrointestinal toxicities combined with low bioavailability of oral preparations (4,6).
CLR127 (18-(p-127I-iodophenyl) octadecyl phosphocholine, short: CLR127), is a novel phospholipid ether (PLE) drug specifically designed for intravenous administration. CLR127 is classified as an alkyl phospholipid ether subtype of the APL that broadly targets cancer cells, with little uptake in healthy tissues (8). Tumor-selective uptake and retention has been demonstrated in murine and human cancers cell lines, patient-derived tumor tissues, multiple xenograft models, and ultimately, in cancer patients (9–11), which led to the clinical development of CLR127 as a carrier molecule for radioactive iodine isotopes, suitable for molecular targeted radiotherapy (CLR131, carrying 131I) and diagnostic imaging (CLR124 carrying 124I) (12,13). We have also demonstrated the capacity of CLR131 to augment the growth inhibitory effect of external beam radiation in head and neck cancer model systems (14). Separately, we have shown that CLR127, in doses substantially higher than those used as a carrier molecule for radioactive iodine, is a potent anti-tumor compound and inhibitor of Akt phosphorylation in mouse models of neuroblastoma, in doses that were non-toxic to experimental animals and potentially achievable in patients (10). Given these favorable drug properties and interference of CLR127 with cell survival mechanisms that are involved in radiation damage repair, we systematically investigated the effect of CLR127 in pediatric and adult solid tumor cell lines and murine xenograft models in combination with ionizing radiation.
Materials and Methods
Cell lines and culture
Human pediatric rhabdomyosarcoma (Rh30) and Ewing sarcoma (TC-71) cell lines were obtained in 2015 from the Children’s Oncology Group Cell and Xenograft Repository, Lubbock, TX. The neuroblastoma line SK-N-AS was kindly provided by Dr. Andrew Davidoff, St. Jude Children’s Research Hospital, Memphis, TN; CHLA-20 was provided by Dr. Wayne Warner, Children’s Hospital of Los Angeles, CA. PC-3 (prostate adenocarcinoma). Human mammary duct epithelial cells (MCF10A) were obtained from ATCC. Primary cultures of normal human cells at low passages (normal human skin, HUFI) were kindly provided by Dr. Victoria Browning (University of Wisconsin-Madison) and were maintained in DMEM with essential and non-essential amino acids. SK-N-AS, PC-3 were cultured in RPMI-1640 medium. CHLA-20, Rh30 and TC-71 were cultured in IMDM. MCF10A cells were cultured in complete growth medium (MEBM, Lonza). Media were supplemented with 10% fetal bovine serum (FBS, Gibco-BRL). Authenticity of cell lines not obtained from commercial sources within 12 months prior to start of the experiments was verified by genomic short tandem repeat (STR) profiling (UW-Madison Pathology Core Laboratory). Periodic PCR and HEK-Blue LPS testing (Invivogen) indicated the cells were free of bacterial contaminants including Mycoplasma species.
CLR127 and derivatives
Clinical-grade CLR127 (former designations: NM404, CLR1404) and the fluorescent derivative for in vitro assays (CLR1501) were kindly provided by Cellectar Biosciences, Madison, WI. Synthesis of CLR127 and fluorescent derivatives has been described previously (8,9).
CLR127-BODIPY (CLR1501) uptake in vitro
Drug uptake was determined by flow cytometry using the fluorescent derivative CLR1501 (CLR127 conjugated to BODIPY-FL) as we have previously published (10) as a surrogate. In brief, tumor or normal cells (5×105/ml) were treated for 20 hours with 5μM CLR1501 in culture medium containing 2% FBS and then washed twice. DAPI (AnaSpec) was added for live cell determination and cells subsequently evaluated on a MACSQuant analyzer (Miltenyi Biotec), followed by data analysis using FlowJo 9.3 software (FlowJo LLC), gating on live, single cells according to standard flow cytometry protocols. Mean fluorescence intensity (MFI) per cell was corrected for cell size by normalizing for autofluorescence variations (15). The unpaired t-test was used to analyze CLR1501 uptake after flow cytometry. All p-values were two-sided and p ≤ 0.05 was used to define statistical significance.
Cell proliferation – MTT Assay
The assay was performed as previously described (16). Linear growth was determined for all cells. Cells were treated for 24 h with CLR127 in concentrations ranging from 0–30 μM, in triplicates. The assay was performed according to the manufacturer’s instructions (Sigma-Aldrich). Absorbance at 570nm was read on a Spectramax Plus microplate reader (Molecular Devices). 0.8% formaldehyde was used as control of total cell death. Live cell equivalents were determined from standard curves, and calculated as percentage from 100% cell growth of the wells treated with excipient.
Clonogenic survival assay
In vitro clonogenic survival following radiation was defined as the ability of single cells to maintain their clonogenic capacity and expand to colonies of ≥ 50 cells, as described previously (17). In brief, cells from single cell suspensions were counted, and seeded in 6-well or 60 mm culture dishes under normal culture conditions for colony formation. Numbers of cells seeded and plate sizes were empirically selected to allow for formation of distinct colonies. Cells were treated for 20 hours with CLR127 in different concentrations (5, 7.5 or 10 μM) or excipient. The drug was removed by washing and standard medium was added. Irradiation (0–6 Gy) was performed in a 137Cs-irradiator (JL Shepherd). Cells were incubated for 10–14 days before fixation and staining of paired sample sets using 0.5% crystal violet in methanol, and gentle aqueous destaining. Colonies were manually counted under magnification. The surviving fraction was determined as the total number of colonies formed divided by the total number of cells seeded, with the survival fraction at 0 Gy normalized to 100%. The clonogenic survival curve for each condition was fitted to a linear quadratic model and compared utilizing the extra sum-of-squares F test (GraphPad Prism 5.01) as previously described, and the cell survival enhancement ratio (ER) calculated as ratio of the mean inactivation concentration after drug exposure as described by Morgan(18,19). Values significantly >1 indicate radiosensitization.
Quantification of γH2AX foci by immunofluorescence microscopy
Cells were cultured on coverslips (18mm × 18 mm) overnight, treated with or without 7.5 μM CLR127 for 20 hours and then irradiated with X-rays (4Gy), harvested immediately or after 1 or 24 hours. The cells were fixed with 4% paraformaldehyde for 10 min, permeabilized in 0.5% Triton-X-100 for 5 min, and then washed in 0.2% Tween/PBS solution. After incubating in blocking solution (normal goat serum and fish gelatin in 0.2% Tween/PBS), the cells were stained with anti-γH2AX antibody (Millipore) overnight at 4°C, and then stained with Alexa Fluor 555 (Invitrogen) and DAPI (Invitrogen). The slides were imaged on a Leica TCS WLL SP8 Laser scanning confocal microscopy (Leica Microsystems) using a 63X oil immersion objective, and the foci were counted using a custom MATLAB program (R2017b, MathWorks). For each treatment condition, the number of γH2AX foci was counted in at least 100 individual cells, and the two-sided t-test was performed to determine the significance between samples treated with or without CLR127 at the same time point.
Western blotting
After treatment of cells at a density of 106 cells/ml with 7.5 μM CLR127 for 20 hours, cells were treated with 5 Gy irradiation and harvested immediately before irradiation or after the indicated times. Cultured cells were lysed at 4°C for 30 min in lysis buffer (20 mM Hepes, pH 7.4, 2 mM EGTA, 50 mM glycerol phosphate, 1% Triton X-100, 10% glycerol, 1 mM dithiothreitol) containing protease and phosphatase inhibitor (Thermo Scientific). Immunoblotting was carried out as previously described (10). Membranes were probed with specific primary antibodies (Supplementary Table 1) and corresponding HRP-conjugated secondary antibodies. Immunoreactivity was visualized with the ECL2 Western Blotting Substrate (Pierce) and analyzed on a Syngene G:BOX Chemi SS-6 gel image analysis system (Syngene). Quantification was performed using ImageJ software (NIH Research Services Branch), and calculated relative to loading control. Data were generated from three independent experiments and statistically analyzed using Student’s two-tailed t-test in MS Excel, and data presented in bar graph format.
Radiation response in human tumor xenografts
Animal experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison in compliance with NIH guidelines. Mice were housed under aseptic conditions. 200 μl cell suspension containing 2×106 tumor cells (> 95% viability, passage 5–25) was inoculated subcutaneously into the dorsal flanks of 6–8 week-old athymic nude mice (CrTac:NCr-Foxn1nu; Taconic). Tumor size was monitored twice weekly by direct measurement with calipers. Tumor volume was calculated using the formula: (π/6) × (largest diameter) × (perpendicular diameter)2. Once average tumor volumes reached 150–200 mm3, mice were assigned by tumor volume to experimental groups so that each cohort contained animals with similar tumor volume distribution and equivalent group means, as calculated in Excel. 10 mg/kg CLR127 or excipient, respectively, was administered via intravenous tail vein injection starting 3 days prior to radiation treatment (XRT) and continued during radiation treatment at the specified intervals. Radiation treatment was delivered by a 320 kVp orthovoltage energy X-ray biological irradiator (X-RAD 320, Precision X-Ray Inc.). Mice were immobilized using custom-designed lead jigs that exposed the tumor-bearing dorsal flank to radiation while minimizing the exposure of non-tumor-bearing normal tissue. Tumor volumes continued to be monitored as above, and general animal health monitored per institutional guidelines. Tumor growth from CLR127 xenograft experiments was analyzed and compared between experimental conditions using a linear mixed effects model with animal specific random effects (to account for repeated measures for the same animals) and the log-transformed tumor volume as the outcome measure (20), using SAS software (SAS Institute).
Immunohistochemistry
Mice bearing PC-3 human prostate carcinoma flank xenografts (n=4/group) were treated with CLR127 (30 mg/kg x 1 dose), XRT (8 Gy x 1 fraction), the combination of CLR127 and XRT or excipient only (control). CLR127 or excipient were injected intravenously 72 hours prior to radiation administration to allow for maximal tumor uptake (9). Tumors were harvested 24 hours after radiation, fixed in 10% neutral-buffered formalin and paraffin-embedded. Sections were prepared for IHC as described previously (21). In brief, slides were incubated at 4°C overnight with primary antibody against γH2AX (Novus Biologicals), followed by a 60-minute incubation with biotin-labeled secondary antibody. Slides were then incubated with streptavidin peroxidase, counterstained with hematoxylin and visualized using the DakoCytomation Liquid DAB+Substrate Chromogen System (Dako). Twelve randomly selected visual fields for each treatment cohort were quantified by counting all γH2AX positive-stained nuclei and determining their proportion to the total number of nuclei. Differences between groups were statistically analyzed using the two-sided t test.
Results
Tumor-selective uptake of CLR127 in tumor cell lines
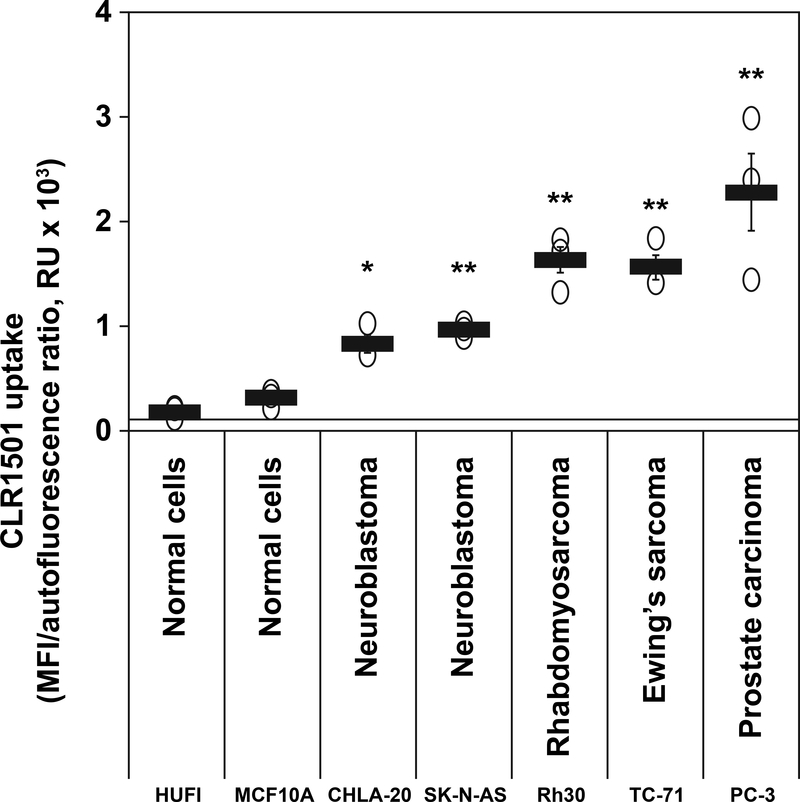
Using the fluorescently labeled derivative CLR1501 as surrogate for CLR127, cellular sequestration and retention in vitro was evaluated by flow cytometry. CLR1501 demonstrated significantly higher uptake and retention in all cancer lines tested (neuroblastoma: CHLA20; SK-N-AS; rhabdomyosarcoma: Rh-30; Ewing sarcoma: TC-71; and prostate carcinoma: PC-3) when compared to uptake in normal human fibroblasts (HUFI) and normal human mammary gland epithelial cells (MCF10A) (p ≤ 0.05), (Fig. 1).
Figure 1.
Uptake of CLR1501 in cancer cells compared to normal cells. Flow cytometry of the uptake of CLR1501 (a CLR127 fluorescent analog) by normal cells (HUFI, human skin fibroblasts; MCF10A, human mammary gland epithelial cells) and tumor cell lines. Averages ± standard error (SE) from three repeats per cell type. *p ≤ 0.05, ** p ≤ 0.01 tumor cells versus normal cells. RU=relative units.
CLR127 decreases cell proliferation in a concentration-dependent manner in tumor cells in vitro
In standard MTT assays, CLR127 treatment for 24 hours, particularly in concentrations above 5 μM, led to significantly lower percentage of proliferating cells (Supplementary Fig. 1) in all tumor cell lines, when compared to controls. After treatment with very high CLR127 concentrations (≥15 μM), impaired proliferation was also observed in normal fibroblasts.
CLR127 treatment increases tumor cell radiosensitivity
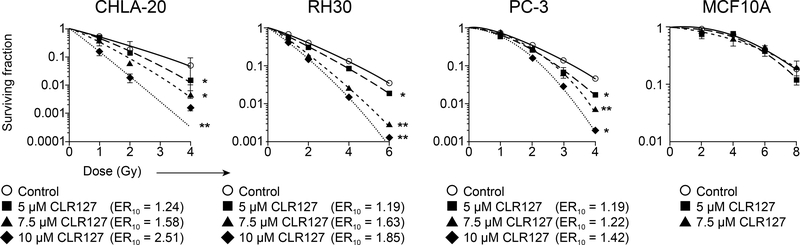
We next explored whether CLR127, beyond direct cytotoxicity to cancer cells, would enhance the effects of radiation. We performed standard in vitro clonogenic survival assays using increasing drug concentrations prior to graded radiation doses. To account for cytotoxic effects of CLR127 as observed by MTT assay, the survival fraction in the absence of radiation was normalized to 100% in the clonogenic assay. As shown in Fig. 2, pretreatment with CLR127 led to concentration-dependent radiosensitization. Radiation dose enhancement ratios for survival at 10% (ER10) ranged from 1.19 to 2.51. We observed statistically significant survival differences at low XRT doses in all cell lines tested. CLR127 pretreatment did not lead to enhanced radiosensitivity in normal MCF10A cells. Cytotoxicity relative to plating efficiency is shown in Supplementary Table 2. Ewing sarcoma lines could not be evaluated with this assay for technical reasons, since treatment of several tested Ewing sarcoma lines with CLR127 and radiation led to failure of the cell lines to reattach to the culture dishes.
Figure 2.
Clonogenic survival of cancer (CHLA-20, Rh30, PC-3) and normal cells (MCF10A) exposed to various XRT doses, with or without CLR127 pretreatment in increasing concentrations as indicated. Radiosensitization of tumor cells by CLR127. Data points represent mean values, bars indicate standard deviation. Enhancement ratios (ER10) as shown. * p ≤ 0.05; ** p ≤ 0.001.
Combining CLR127 and Radiation Affects DNA Damage Response
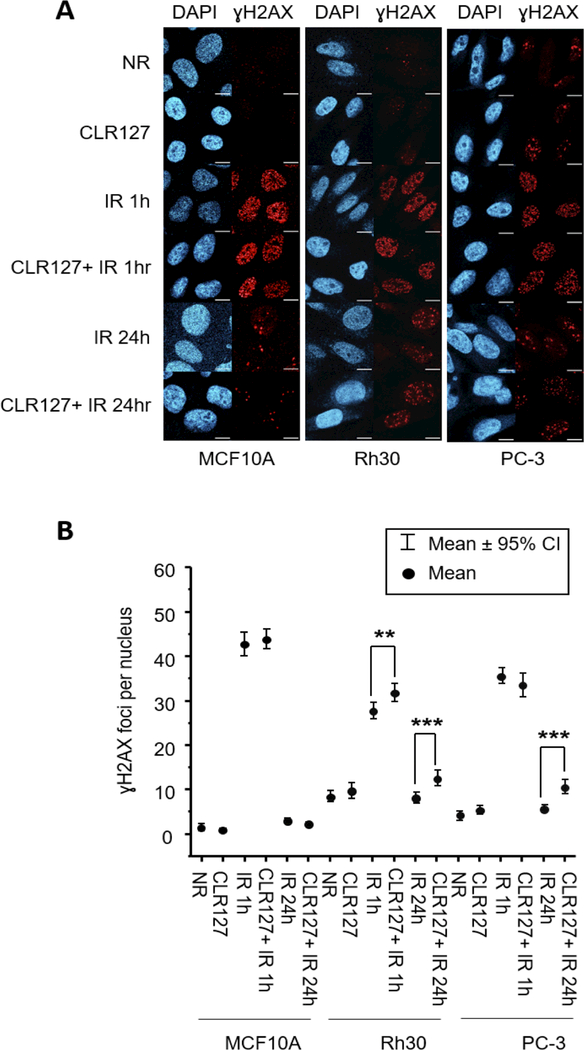
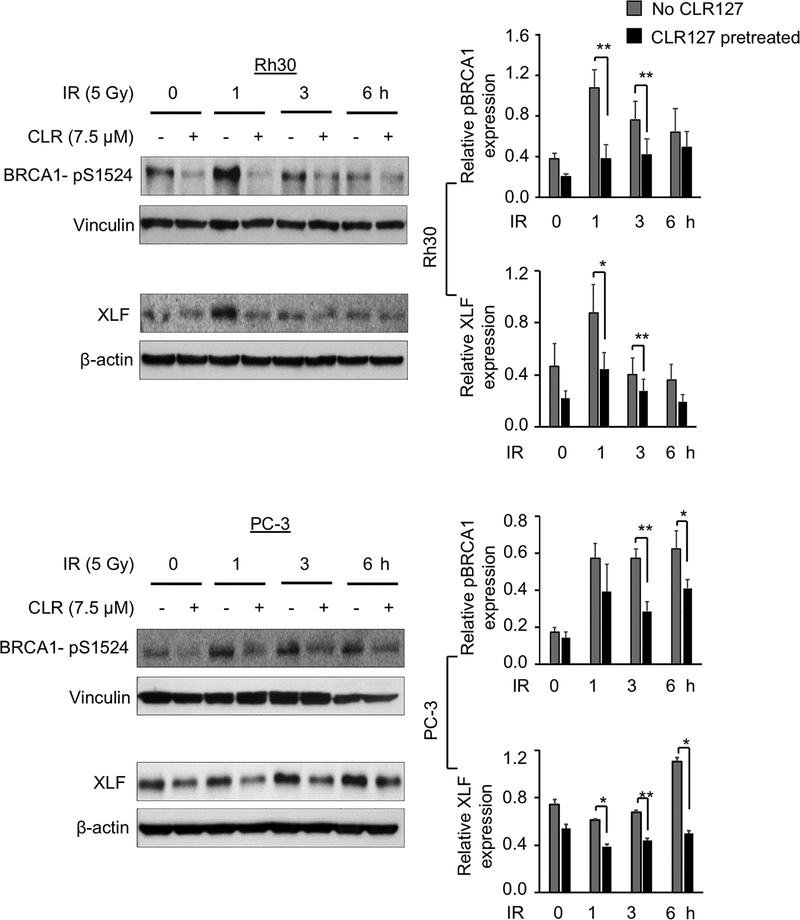
The impact of CLR127 and radiation on double-stranded DNA (dsDNA) breakage and repair was evaluated by confocal microscopy of γH2AX foci and western blot experiments (Fig. 3A and 3B and Supplementary Fig. 2). While normal cells (MCF10A) treated with both CLR127 and XRT exhibited steady state γ-H2AX foci formation at 1h and activation of DNA damage response (DDR) signaling pathway by 24h, cancer cells Rh30 and PC-3 showed sustained γ-H2AX foci formation up to 24h, confirmed by statistical analysis (Fig. 3A and 3B and Supplementary Fig. 2). To assess the recruitment of other proteins to γ-H2AX foci, we compared the relative expression of BRCA1 and XLF in tumor cells exposed to radiation with or without CLR127 pretreatment up to 6 hours post treatment (Fig. 4). In PC-3 cells, p-BRCA1 and XLF were expressed at consistent level, whereas in Rh30 cells, p-BRCA1 (pS1524) phosphorylation increased and XLF expression decreased. Control irradiated cells followed the expected pattern of reduced recruitment, consistent with γ-H2AX foci patterns (Fig. 3A and 3B).
Figure 3.
Immunofluorescence microscopy was performed with anti-γH2AX antibody to visualize the DNA repair process (Fig. 3A, upper panel, scale bar: 10 μm). The data points (Fig. 3B, lower panel) represent mean values of γH2AX foci per nucleus, while the error bars show 95% confidence intervals. With the combined treatment of IR and CLR127, quantification of γH2AX foci revealed there are significant increase of mean foci count in cancer cell lines Rh30 and PC-3 at 24 hours compared to the samples treated with IR only (** p<0.01, *** p<0.001). Normal cell MCF10A did not show significant changes in foci number across all time points. NR: no irradiation; IR: irradiated.
Figure 4.
Effect of CLR127 (CLR) and radiation on DNA damage repair proteins pBRCA1 and XLF in vitro. Cells were treated with or without 7.5 μM CLR for 16 hours and exposed to 5 Gy radiation. Cells were collected directly before radiation (0 h), and at 1, 3, and 6 hours (h) and subjected to western blotting. The obtained bands were quantified relative to β-actin (XLF) or vinculin (pBRCA1) loading control using ImageJ software. Left row: Representative western blots shown. Right: Wide bars show mean relative pBRCA1 and XLF expression, respectively. Bars show mean values of three independent experiments, small bars depict standard error. Statistical differences were calculated with Student’s two-tailed t-test (*p≤0.05; **p≤0.01).
CLR127 augments the therapeutic response to external beam radiation in mice bearing human tumor flank xenografts
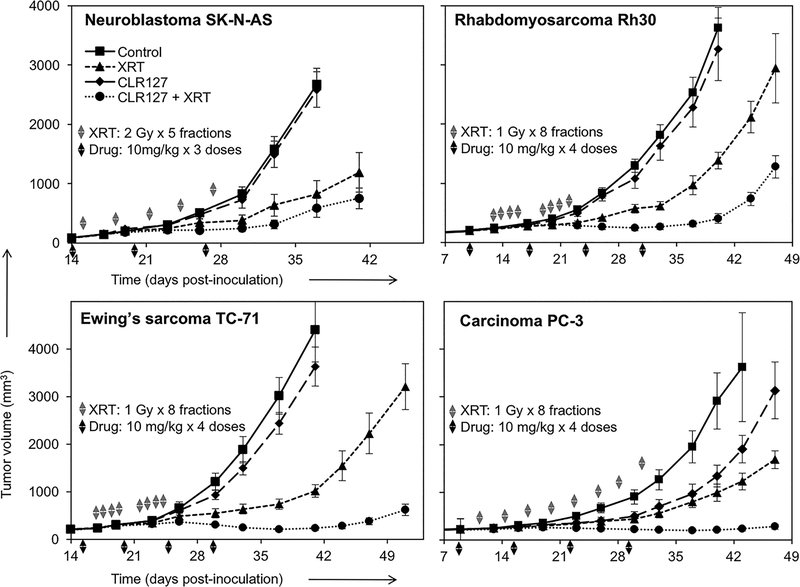
To test whether our in vitro findings, i.e. a significant radiosensitizing effect on various solid tumor lines, could be reproduced in vivo, we evaluated the capacity of CLR127 to augment radiation response in athymic nude mice bearing human flank tumor xenografts. In an effort to capture the interaction between CLR127 and radiation, treatment doses and schedules were based upon pilot experiments such that the tumor response to each individual modality would be modest. We established xenografts using human tumor lines for neuroblastoma (SK-N-AS), rhabdomyosarcoma (Rh30), Ewing sarcoma (TC-71) and prostate adenocarcinoma (PC-3). The combination of CLR127 and fractionated radiation resulted in a significantly increased tumor response (average tumor volumes and growth rates) in the two sarcomas and prostate adenocarcinoma (P < 0.001) and improvement of the already pronounced radiation effect on SK-N-AS (neuroblastoma) when compared to mice that received excipient, CLR127 alone, or radiation alone (Fig. 4, Supplementary Fig. 3 and Supplementary Table 3). Treatment with CLR127 did not lead to discernible adverse effects on overall animal health, consistent with our previously published observations (10). These in vivo data demonstrate a capacity of CLR127 to augment radiation response in vivo in tumors of ectodermal, neuroectodermal and mesenchymal origin.
Treatment with CLR127 leads to increased γH2AX foci in irradiated tumor tissue
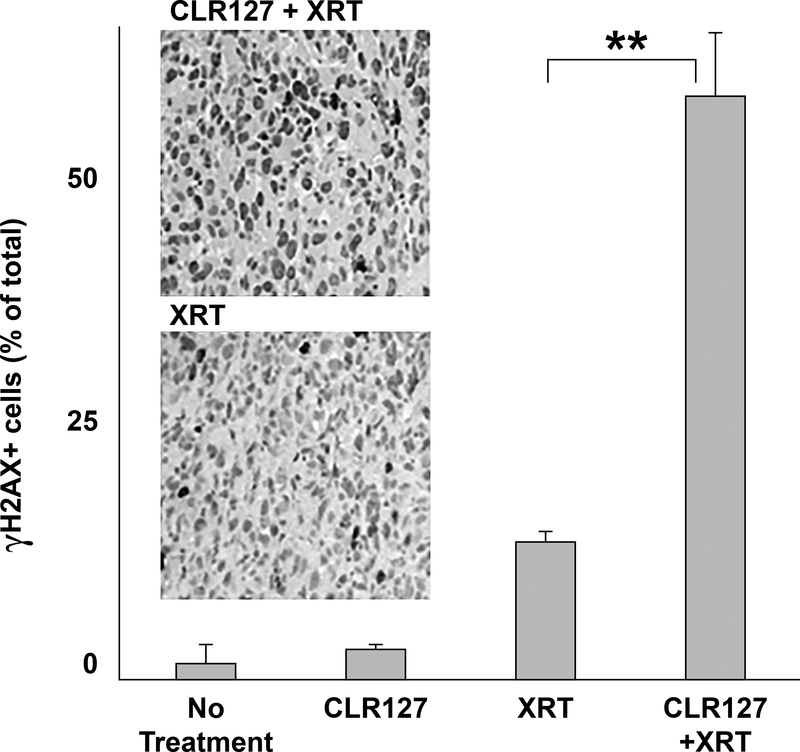
We tested the effect of CLR127 on DNA damage repair after radiation in mice bearing human prostate carcinoma PC-3 xenografts by immunohistochemistry. Tumors of mice that were treated with CLR127 plus XRT demonstrated a significantly higher fraction of γH2AX positive cells 24 hours after radiation treatment compared to tumor tissue of animals that received XRT only or CLR127 only, respectively (p≤0.001), confirming increased dsDNA radiation damage in the combination treatment cohort (Fig. 6).
Figure 6.
Effect of in vivo CLR127 and radiation on DNA damage repair. Mice bearing human prostate carcinoma PC-3 xenografts were treated with CLR127 (30 mg/kg/dose x 1 dose), XRT (8 Gy x 1 fraction) or the combination of CLR127 and XRT. CLR127 was injected intravenously 72 hours prior to radiation administration to allow for maximal uptake. Tumors were harvested 24 hours after radiation. γH2AX expression in the tumor xenograft was examined by immunohistochemistry. Shown is the percentage of γH2AX positive cells obtained from average of twelve visual fields randomly selected from three tumors per each treatment condition: excipient control/no treatment; CLR127 only; radiation only (XRT); and radiation and CLR127 treatment. Bars represent standard error. Panels: examples of visual fields under microscopic examination (20x magnification). Upper: CLR127 (CLR) and XRT, lower: XRT only. ** p ≤ 0.001.
Discussion
Our experiments demonstrate a remarkable capacity for the tumor-selective phospholipid ether drug CLR127 to sensitize several human solid tumor cell lines to XRT in vitro and human tumor xenografts in mouse models. Importantly, in vivo radiosensitization occurred at therapeutically achievable doses of this intravenously administered compound.
Many of the drugs that are being used to sensitize tumor cells to ionizing radiation are non-tumor-specific agents. They are often given concomitantly with radiation (chemoradiation) to achieve synergy rather than being used for radiosensitization only. Since these drugs are non-targeted, their application can be limited due to their own side-effect profile and toxicity to normal tissues (22). Our data suggest that tumor-targeted radiosensitization might be accomplished with CLR127 in the tumor types we have tested. Since CLR127 is a compound shown to be taken up by more than 60 different pediatric and adult cancer cell lines with high selectivity, patient-derived tumor cells and, as recently demonstrated, brain tumor initiating cells (9,10), it is reasonable to believe that many more tumor types might be susceptible to radiosensitization with CLR127.
We chose cell lines of several tumors in which radiotherapy plays a particularly important role, namely prostate adenocarcinoma and the pediatric malignancies neuroblastoma, rhabdomyosarcoma and Ewing sarcoma. In vitro, utilizing the fluorescent derivative CLR1501 as surrogate, we demonstrate significantly higher uptake and retention in cancer cells when compared to normal cells (Fig. 1). This is in alignment with previous data reported by us and others showing almost universal significant uptake and retention of CLR127 and its fluorescent or radiolabeled derivatives in a variety of cancer cells in vitro and in vivo, with little uptake in pre-malignant or normal cells or tissues (9,10,23). Our data also demonstrate significant reduction of cell proliferation in prostate carcinoma, Ewing’s sarcoma and rhabdomyosarcoma cell lines in vitro after treatment with CLR127, which were significant in concentrations ≥ 7.5 μM for all tested cell lines (see Supplementary Fig. 1), concordant with previously reported results in various neuroblastoma cell lines (10).
We next evaluated the in vitro radiosensitizing effect of CLR127 to ionizing radiation in standardized clonogenic survival assays (Fig. 2), and demonstrated significant radiosensitization in PC-3, Rh30 and CHLA-20 cell lines. After normalizing for cell killing by CLR127 alone, the radiosensitizing effect was already apparent and statistically significant at low drug concentrations and radiation doses. Radiosensitizing effect was consistently concentration-dependent, and radiation enhancement ratios (ER10) ranged between modest 1.19 and robust 2.51. No differences in the clonogenic capacity were seen in MCF10A cells, reflecting lack of effective drug uptake in normal cells.
To further investigate effects of CLR127 on DNA damage responses, we performed confocal microscopy and western blot experiments. Quantification of γ-H2AX as a function of time serves as a readout for both DNA double stranded breaks (early response) and activation of DNA damage response (DDR) signaling pathway (late response) (24). We observed sustained γ-H2AX foci up to 24 hours in cancer cells, confirming increased radiosensitization of CLR127 in Rh30 and PC-3 cells. Normal cells (MCF10A) treated with both CLR127 and XRT exhibited steady state γ-H2AX foci formation at 1h and DDR by 24h. Taken together, these experiments suggest the potential of CLR127 mediating the activation of DDR signaling pathways. Towards dissecting the role of CLR127 in recruiting other proteins to the γ-H2AX foci, we evaluated the role of BRCA1 – an intermediate or late protein involved in DSB repair and XLF, a bona fide classical nonhomologous end-joining (c-NHEJ) factor, in the presence of ionizing radiation in biochemical experiments. Intriguingly, quantitative analyses of western blots up to 6 hours in Rh30 cells revealed opposing trends of potential recruitment of these proteins to γ-H2AX foci (Fig. 4) in combined CLR127 and radiation treatments, with increased phospho-BRCA1 (pS1524) and decreased XLF protein expression respectively. Control irradiated cells followed an expected pattern of reduced p-BRCA1 and XLF recruitment, consistent with γ-H2AX foci pattern (Fig. 3A and 3B). In contrast, in PC-3 cells both p-BRCA1 and XLF proteins were expressed at consistent level. These biochemical experiments suggest that early events of c-NHEJ are primarily driven by p-BRCA1 at the γ-H2AX foci or DSB site while the fraction of XLF, recruited at 1h at the γ-H2AX foci, appears to dissociate over time (Fig. 4) in Rh30 cells. Taken together, both imaging of γ-H2AX foci and biochemical experiments of p-BRCA1 and XLF indicate that combined CLR127 and radiation have cell type specificity in terms of engaging the DSB repair and c-NHEJ processes. Further research is warranted to better understand the influence of CLR127 on DNA damage sensing and repair mechanisms, which may help guide in development and design of efficacious clinical protocols.
Our in vivo experiments investigated the effect of CLR127 on fractionated XRT in mice bearing human flank xenografts. A significant radiosensitizing effect on tumor growth was demonstrated in nude mice bearing PC-3, Rh30, and TC-71 xenografts (p ≤ 0.001), with enhanced effects on SK-N-AS as well, although not statistically significant in the context of our treatment regimen (Fig. 5). In PC-3 tumors, harvested 24 hours after the end of a combination treatment with CLR127 and fractionated XRT, γH2AX expression in tumor cells was significantly higher than in the tumors treated with either modality alone (Fig. 6, p ≤ 0.001), suggesting a profound inhibitory effect of CLR127 on irradiated cells to successfully repair dsDNA damage. Radiation treatment doses and fractionations for our tumor models were chosen based on pilot experiments to determine dose-effect relationships and differed between xenograft models. CLR127 dosing was based on extensive pre-clinical toxicity studies in rodents and cynomolgus monkeys, as well as our own data, in which repetitive intravenous dosing up to 30 mg/kg once weekly for up to 7 weeks was safe, and with no adverse effects observed in the experimental animals (10).
Figure 5.
CLR127 augments radiation response in xenografted pediatric cancers and adult prostate adenocarcinoma. Nude mice bearing human xenografts were treated with XRT (dashed line with triangles), CLR127 (solid line with diamonds), or both modalities (dotted line with solid circles); control/untreated mice: solid line with squares. Symbols denote mean tumor volume ± SE. Gray arrowheads indicate fractionated radiation. Black arrowheads indicate intravenous injection of CLR127. Drug (CLR127) and XRT doses are indicated in the figures. n=10–12 xenografts per group. Combination treatment vs. XRT alone p ≤ 0.001 in all tumors except SK-N-AS (not significant).
In summary, our data suggest that the alkyl phospholipid ether analog CLR127 is a potent tumor-selective radiosensitizer for a variety of cancer types in preclinical studies and provides a strong rationale for clinical testing with standard radiotherapy regimens.
Future research should address whether the effects we observed in our tumor models can be further extended to additional tumor types. Given the almost universal and selective uptake of CLR127 in malignant cells and the intravenous formulation that is paired with a high therapeutic index in pre-clinical toxicity studies, CLR127 may afford a distinct and clinically valuable advantage over other commonly utilized radiosensitizing agents.
Supplementary Material
Acknowledgements
We thank Simran Kaur and Elaine Dandan for help with multiple biological assays and Dylan Wiese for his help with the immunofluorescence experiments.
Financial Support: This work was supported in part by NIH R21CA198392–01 (to M.Otto and B.P.Bednarz), NIH/NCI P30CA014520 (UW-Carbone Cancer Center Support Grant), NIH P50 DE026787 (UW-Head and Neck SPORE, to P.M. Harari), Midwest Athletes against Childhood Cancer Foundation (to M.Otto), Hyundai Hope on Wheels Foundation (to M.Otto), and Young Investigator Sub-Award from SU2C/St. Baldrick’s Pediatric Dream Team Translational Research Grant SU2C-AACR-DT1113 (to M.Otto).
Footnotes
Disclosure of Potential Conflict of Interest: There are no competing interests to declare.
References
- 1.Wardman P Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol) 2007;19(6):397–417 doi 10.1016/j.clon.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 2.van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Current pharmaceutical design 2008;14(21):2061–74. [DOI] [PubMed] [Google Scholar]
- 3.Vink SR, van der Luit AH, Klarenbeek JB, Verheij M, van Blitterswijk WJ. Lipid rafts and metabolic energy differentially determine uptake of anti-cancer alkylphospholipids in lymphoma versus carcinoma cells. Biochemical pharmacology 2007;74(10):1456–65 doi 10.1016/j.bcp.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 4.van Blitterswijk WJ, Verheij M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochimica et biophysica acta 2013;1831(3):663–74 doi 10.1016/j.bbalip.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Rios-Marco P, Marco C, Galvez X, Jimenez-Lopez JM, Carrasco MP. Alkylphospholipids: An update on molecular mechanisms and clinical relevance. Biochimica et biophysica acta 2017;1859(9 Pt B):1657–67 doi 10.1016/j.bbamem.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Vink SR, van Blitterswijk WJ, Schellens JH, Verheij M. Rationale and clinical application of alkylphospholipid analogues in combination with radiotherapy. Cancer Treat Rev 2007;33(2):191–202 doi 10.1016/j.ctrv.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Alkyl-lysophospholipids activate the SAPK/JNK pathway and enhance radiation-induced apoptosis. Cancer Res 1999;59(10):2457–63. [PubMed] [Google Scholar]
- 8.Pinchuk AN, Rampy MA, Longino MA, Skinner RW, Gross MD, Weichert JP, et al. Synthesis and structure-activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogues. J Med Chem 2006;49(7):2155–65 doi 10.1021/jm050252g. [DOI] [PubMed] [Google Scholar]
- 9.Weichert JP, Clark PA, Kandela IK, Vaccaro AM, Clarke W, Longino MA, et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci Transl Med 2014;6(240):240ra75 doi 10.1126/scitranslmed.3007646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marino R, Baiu DC, Bhattacharya S, Titz B, Hebron E, Menapace BD, et al. Tumor-selective anti-cancer effects of the synthetic alkyl phosphocholine analog CLR1404 in neuroblastoma. Am J Cancer Res 2015;5(11):3422–35. [PMC free article] [PubMed] [Google Scholar]
- 11.Baiu DC, Marsh IR, Boruch AE, Shahi A, Bhattacharya S, Jeffery JJ, et al. Targeted Molecular Radiotherapy of Pediatric Solid Tumors Using a Radioiodinated Alkyl-Phospholipid Ether Analog. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2018;59(2):244–50 doi 10.2967/jnumed.117.193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grudzinski JJ, Titz B, Kozak K, Clarke W, Allen E, Trembath L, et al. A phase 1 study of 131I-CLR1404 in patients with relapsed or refractory advanced solid tumors: dosimetry, biodistribution, pharmacokinetics, and safety. PloS one 2014;9(11):e111652 doi 10.1371/journal.pone.0111652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubner SJ, Mullvain J, Perlman S, Pishvaian M, Mortimer J, Oliver K, et al. A phase 1, multi-center, open-label, dose-escalation study of 131I-CLR1404 in subjects with relapsed or refractory advanced solid malignancies. Cancer Invest 2015;33(10):483–9 doi 10.3109/07357907.2015.1081691. [DOI] [PubMed] [Google Scholar]
- 14.Morris ZS, Weichert JP, Saker J, Armstrong EA, Besemer A, Bednarz B, et al. Therapeutic combination of radiolabeled CLR1404 with external beam radiation in head and neck cancer model systems. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2015;116(3):504–9 doi 10.1016/j.radonc.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzur A, Moore JK, Jorgensen P, Shapiro HM, Kirschner MW. Optimizing optical flow cytometry for cell volume-based sorting and analysis. PloS one 2011;6(1):e16053 doi 10.1371/journal.pone.0016053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol 2011;716:157–68 doi 10.1007/978-1-61779-012-6_9. [DOI] [PubMed] [Google Scholar]
- 17.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res 2013;73(15):4791–800 doi 10.1158/0008-5472.can-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimple RJ, Vaseva AV, Cox AD, Baerman KM, Calvo BF, Tepper JE, et al. Radiosensitization of epidermal growth factor receptor/HER2-positive pancreatic cancer is mediated by inhibition of Akt independent of ras mutational status. Clin Cancer Res 2010;16(3):912–23 doi 10.1158/1078-0432.ccr-09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan MA, Parsels LA, Kollar LE, Normolle DP, Maybaum J, Lawrence TS. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res 2008;14(16):5142–9 doi 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38(4):963–74. [PubMed] [Google Scholar]
- 21.Li C, Huang S, Armstrong EA, Francis DM, Werner LR, Sliwkowski MX, et al. Antitumor Effects of MEHD7945A, a Dual-Specific Antibody against EGFR and HER3, in Combination with Radiation in Lung and Head and Neck Cancers. Mol Cancer Ther 2015;14(9):2049–59 doi 10.1158/1535-7163.mct-15-0155. [DOI] [PubMed] [Google Scholar]
- 22.Bartelink H, Schellens JH, Verheij M. The combined use of radiotherapy and chemotherapy in the treatment of solid tumours. Eur J Cancer 2002;38(2):216–22. [DOI] [PubMed] [Google Scholar]
- 23.Deming DA, Maher ME, Leystra AA, Grudzinski JP, Clipson L, Albrecht DM, et al. Phospholipid ether analogs for the detection of colorectal tumors. PloS one 2014;9(10):e109668 doi 10.1371/journal.pone.0109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo LJ, Yang LX. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In vivo 2008;22(3):305–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.