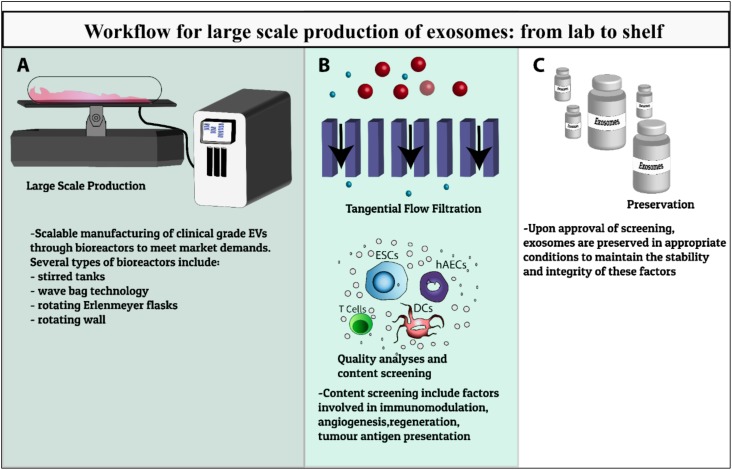
FIGURE 1.
Workflow summary of EVs production for clinical use. Schematic of the development of EV therapeutics from preclinical testing to scalable bioprocesses including (A) development of large scale manufacturing of clinical grade EVs through various types of bioreactors, (B) characterization, quality analysis and content screening including factors involved in immunomodulation, angiogenesis, regeneration, tumor antigen presentation, (C) preservation in appropriate storage conditions to maintain the stability and integrity of these factors to meet clinical-scale demands.