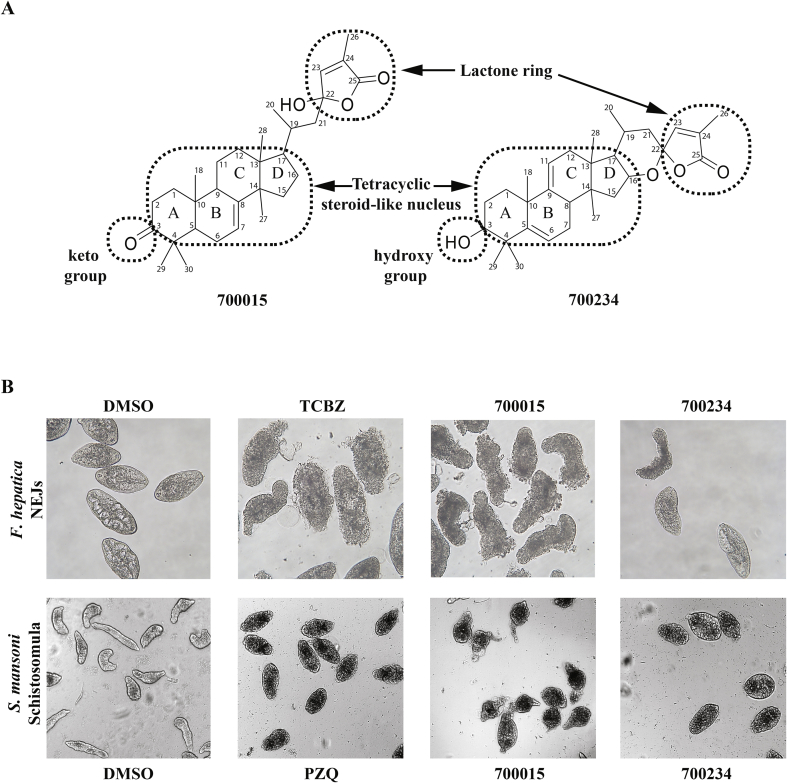
Fig. 3.
A lactone ring is associated with the dual anthelmintic activity of the two most effective triterpenoids. (A) The structural elucidation of triterpenoids 700015 and 700234 revealed a tetracyclic steroid-like nucleus core, a keto- (700015) or hydroxyl- (700234) group and a conserved lactone ring. ‘A, B, C, D’ represent the classic lettering system for steroids (Moss, 1989). Carbon numbering is derived from the 13C NMR structural elucidation of each triterpenoid. (B) Representative images of F. hepatica NEJs co-cultivated with DMSO (0.1%, negative control), TCBZ (10 μM in 0.1% DMSO, positive control), 700015 (10 μM in 0.1% DMSO) and 700234 (10 μM in 0.1% DMSO) obtained by bright field microscopy (20× objective) compared to representative images of S. mansoni schistosomula co-cultivated with DMSO (0.625%, negative control), PZQ (10 μM in 0.625% DMSO, positive control), 700015 (10 μM in 0.625% DMSO) and 700234 (10 μM in 0.625% DMSO) obtained by the high content imaging platform (10× objective). All parasite images were acquired at 72 h post parasite/compound co-cultivation.