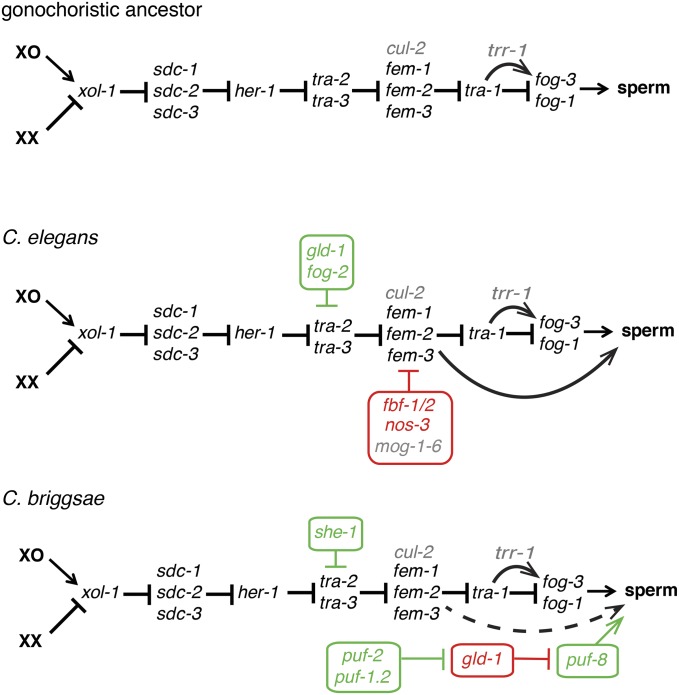
Figure 3.
Convergent evolution of self-fertility via distinct changes alterations of germline sex determination. The core body-wide sex determination pathway (black), which acts in all dimorphic tissues, is shared with outcrossing relatives (top). Upstream factors that sense X dosage and regulate both sex determination and dosage compensation (xol-1 and the sdc genes), are not depicted here for simplicity. The XX hermaphrodites of C. elegans (middle) and C. briggsae (bottom) both produce sperm in an otherwise female body by germline-specific modification of sex determination. Germline-specific factors that promote sperm production in each are indicated in green, while those limiting it are in red. Note that in the C. briggsae case, the influence of she-1 on tra-2 is indirect, and the action of the pathway consisting of puf-2, puf-1.2, gld-1, and puf-8 has not yet been placed along the global pathway, and is thus conservatively depicted as a parallel pathway. Pleiotropic accessory factors with important roles in sexual fate are indicated in gray. The alternative functions of homologous genes and the role of species-specific genes in both hermaphrodites are particularly noteworthy. The arrows connecting fem genes directly to sperm fate in C. elegans depicts how loss of any of the fem genes phenotypically feminizes tra-1 germ cells without loss of fog-3 expression (Chen and Ellis 2000). In C. briggsae, a similar result is found for fem-3, but not fem-2, and the effect is to convert the mostly male tra-1 germ line to a consistent hermaphroditic (rather than female) pattern (Hill and Haag 2009). For this reason, the equivalent arrow is dashed.