Significance
Inguinal hernia is one of the most common disorders that affect elderly men. A major pathology underlying inguinal hernia is the fibrosis and other degenerative changes that affect the lower abdominal muscle strength adjacent to the inguinal canal. Here we describe a critical role of estrogen and its nuclear receptor that enhance fibroblast proliferation and muscle atrophy, leading to inguinal hernia. Further research may reveal a potential role of estrogen ablation to prevent muscle fibrosis or hernia in a subset of elderly men.
Keywords: aromatase, estrogen receptor-α, androgen, fibrosis, hernia
Abstract
Inguinal hernia develops primarily in elderly men, and more than one in four men will undergo inguinal hernia repair during their lifetime. However, the underlying mechanisms behind hernia formation remain unknown. It is known that testosterone and estradiol can regulate skeletal muscle mass. We herein demonstrate that the conversion of testosterone to estradiol by the aromatase enzyme in lower abdominal muscle (LAM) tissue causes intense fibrosis, leading to muscle atrophy and inguinal hernia; an aromatase inhibitor entirely prevents this phenotype. LAM tissue is uniquely sensitive to estradiol because it expresses very high levels of estrogen receptor-α. Estradiol acts via estrogen receptor-α in LAM fibroblasts to activate pathways for proliferation and fibrosis that replaces atrophied myocytes, resulting in hernia formation. This is accompanied by decreased serum testosterone and decreased expression of the androgen receptor target genes in LAM tissue. These findings provide a mechanism for LAM tissue fibrosis and atrophy and suggest potential roles of future nonsurgical and preventive approaches in a subset of elderly men with a predisposition for hernia development.
Inguinal hernia is a common malady in elderly men, and hernia repair is the most commonly performed general surgical procedure in the United States. Although its pathogenesis is poorly understood, the lifetime risk of inguinal hernia is 27% in men and 3% in women (1). Approximately 800,000 inguinal hernia repairs are performed every year (1, 2), and annual health care costs directly attributable to inguinal hernia exceed $2.5 billion in the United States (3). Surgery is the only treatment option for an inguinal hernia. Unfortunately, complications, such as long-term postoperative pain, nerve injury, wound infection, and recurrence continue to challenge surgeons and patients (4–7). There are no actively utilized animal models for studying inguinal hernia or sex steroid-related muscle fibrosis and atrophy; furthermore, currently there are no experimental or approved medical options for the prevention of inguinal hernias in subsets of elderly men.
Inguinal hernias are termed “indirect” if the bowel is herniated via a defective inguinal ring or “direct” if the bowel protrudes through another weakened portion of lower abdominal muscle (LAM) wall (3, 5). The inguinal canal in male mice, which connects the abdominal cavity and the scrotum, is structurally similar to that in men and particularly vulnerable to indirect herniation of bowel (8). Therefore, experimental scrotal hernias in mice have similarities to indirect inguinal hernia in humans, which comprise two-thirds of all inguinal hernias in men (5). Previous studies in mice suggest that the development of scrotal hernias may be associated with abnormalities in the abdominal muscles, particularly those in the inguinal region (9, 10). In men, histological studies have identified myocyte (myofiber) atrophy, fibrosis, and fatty degeneration in the internal inguinal ring area from indirect hernia patients and in the abdominal wall surrounding a direct hernia border (11–13). LAM tissue is composed of layers of oblique and transverse skeletal muscle made of myocytes. Stromal tissue, a mixture of well-organized fibroblasts and extracellular matrix (ECM), surrounds a single myocyte, fascicles (groups of myocytes), or the entire muscle tissue (groups of fascicles), and eventually becomes the deep fascia. An inguinal hernia occurs if both the muscle and adjacent fascia are weakened and can no longer support bowel in the abdomen.
Age is a significant risk factor for inguinal hernia formation in men with a striking increase in incidence after the age of 55 y. By the time they reach 75 y of age, nearly 50% of men will develop inguinal hernia (14, 15). Indirect inguinal hernia, which comprises 70% of all hernias, peaks between the ages of 70 and 79 y in men (16). It is a disease of primarily elderly men. The increased risk of inguinal hernia in elderly men may be related to age-linked skeletal muscle atrophy associated with fibrosis in the inguinal area. However, the mechanisms mediating skeletal muscle atrophy and fibrosis that increases the risk of hernia formation are not well understood.
Sex steroid hormones change as men age. Age-related changes in serum estradiol (E2) levels in men are conflicting, as some studies have reported increases but others have noted unchanged or even decreased E2 levels with advancing age (17–24). Conversion of circulating testosterone (T) to E2 via aromatase expression in bulky tissues—namely, skeletal muscle and adipose tissue—produces the majority of estrogen in men (25, 26). Human muscle and adipose tissue aromatase expression or activity has been found to increase with advancing age, which coincides with the incidence of inguinal hernia (25, 27–29). In the 1930s, two separate laboratories reported that ∼40% of male mice that received postnatal estrogen injections or ovarian grafts developed scrotal hernias (8, 30). Estrogen injections initiated as early as postnatal day 28 or as late as 30 wk of age led to the development of scrotal hernia within a few weeks (8). These data were suggestive of a direct link between local tissue estrogen and acquired inguinal hernias and do not support the possible contribution of a congenital defect. The underlying cellular and molecular mechanisms, however, remain unknown. Conversely, serum T levels decrease by 20% by age 50 and by 50% by age 80 y in association with decreased skeletal muscle mass in men (31–33). A higher ratio of E2 to T was observed in elderly men compared with younger men (31). It remains unclear whether the age-related shift in the estrogen to T ratio in the LAM tissue causes muscle fibrosis and atrophy and predisposes a subset of older men to develop inguinal hernia.
Aromatase is the only enzyme that catalyzes the conversion of T to E2. The tissue distribution patterns of aromatase expression in humans and mice are markedly distinct. In male mice, aromatase is expressed only in the testes, gonadal fat, and brain via 3 promoters, whereas humans use 10 distinct promoters to express aromatase in many peripheral tissues, including skeletal muscle. We recently generated mice expressing aromatase from the human promoter (Aromhum) to mimic human physiology with respect to aromatase expression and estrogen production (34). Aromhum mice physiologically express the human aromatase gene in many peripheral tissues, including skeletal muscle (25, 35, 36). Intriguingly, male Aromhum mice exhibit increased estrogen levels in peripheral LAM tissues, low serum T levels, and scrotal hernia formation, mimicking what has been observed in a subset of older men. Thus, we used this mouse model to test the hypothesis that alterations in E2, T, and their nuclear receptors, estrogen receptor-α (ERα) and androgen receptor (AR), in LAM tissues lead to fibrosis, skeletal muscle atrophy, and the development of scrotal hernias. We also investigated some of the underlying cellular and molecular mechanisms behind hernia formation.
Results
Humanized Aromatase Expression and Physiological Estrogen Production in LAM Tissues Are Associated with Scrotal Hernia Formation in Aromhum Mice.
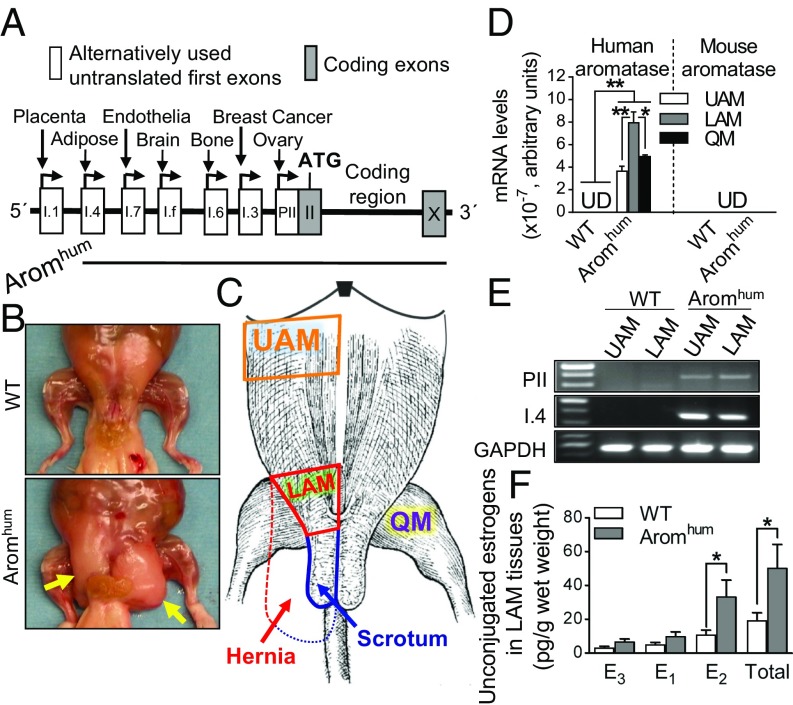
Aromhum mice were generated and characterized as previously described (34). Briefly, we isolated a human BAC clone containing the human aromatase coding sequence flanked by its full-length 93-kb 5′-regulatory region and the 3′-polyadenylation site, and injected the BAC clone DNA into pronuclear mouse embryos to generate transgenic mice with a single copy of the transgene in the germline. We obtained six male transgene-positive founders in two independent male transgenic lines, F1771 and F1772. The Aromhum F1771 line included a >78-kb 5ʹ-flanking region encompassing the distal promoters I.4, I.7, I.f, as well as the proximal cluster of promoters I.6 and I.3/PII (Fig. 1A), whereas the Aromhum F1772 line had a 4.3-kb 5′-flanking region containing only the proximal promoters I.6 and I.3/PII (34). Bilateral scrotal hernias were observed in 90–100% of male Aromhum mice by 12 wk from all two lines established from the founders, whereas none of the WT animals developed any hernias. Because all founder lines showed a similar phenotype, we primarily reported the results obtained from a single founder from the Aromhum F1771 line (referred to as Aromhum in this report). The scrotal hernia sacs contained abdominal viscera, including gonads, gonadal fat, urinary bladder, and bowel (Fig. 1B). In Aromhum mice, LAM tissues became progressively fibrotic and distended, comprising the major part of the hernia wall, contiguous with the scrotum (Figs. 1C and 2 C and D). Human aromatase expression driven by its native promoters in Aromhum mice resembled the human pattern of aromatase expression (SI Appendix, Table S1) (25, 35–37).
Fig. 1.
Human aromatase expression, abdominal muscle tissue estrogen levels, and scrotal hernia formation in Aromhum transgenic mice. (A) Schematic of the human BAC clone construct used to generate Aromhum transgenic mice, which contained all alternatively used promoters with downstream first exons of the aromatase gene except for placental exon I.1. (B) Dissected abdominal musculature of 26-wk-old WT and Aromhum mice. Yellow arrows indicate scrotal hernia. (C) Schematic of mouse abdominal muscle anatomy in WT and Aromhum mice. UAM, LAM, QM, the scrotum, and hernia are shown in the sketch. Solid red line and solid blue line indicate normal LAM tissue and normal scrotum in WT mice, respectively. Dashed red line indicates expanded fibrotic LAM tissue that comprises the hernia wall contiguous with the scrotum (dashed blue line) in Aromhum mice. (D) Human and mouse aromatase mRNA levels were determined in UAM, LAM, and QM of Aromhum mice. UD, undetermined. Statistical analysis by two-way ANOVA with Sidak’s multiple comparison test, *P < 0.05, **P < 0.01, n = 8 mice per group. (E) Exon-specific RT-PCR confirmed that distinct promoters drive human aromatase expression in the abdominal muscle tissues of Aromhum mice. GAPDH mRNA levels served as internal control. Data are representative of three independent experiments. (F) LAM tissue unconjugated estrogens were measured by LC-MS2 assay. E1, E2, and E3 are shown. Two-tailed Student’s t test, *P < 0.05, n = 14 mice.
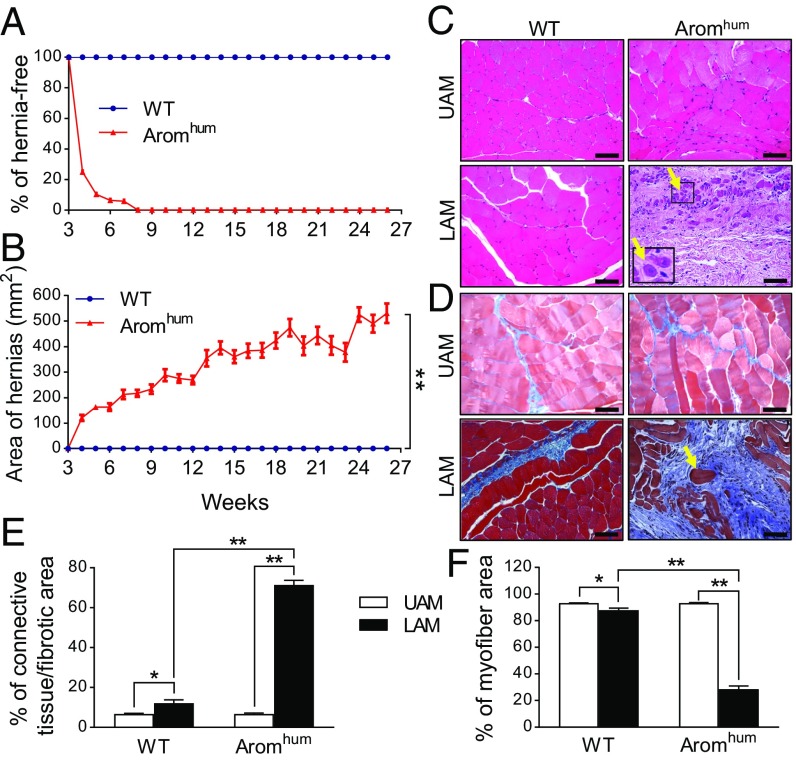
Fig. 2.
Human aromatase expression and estrogen production lead to LAM tissue fibrosis, myocyte atrophy, and the development of scrotal hernia in Aromhum mice. (A) The incidence of scrotal hernia. Scrotal hernia formation was monitored by weekly visual inspection from 3 to 26 wk of age. No scrotal hernia was seen in the WT group. n = 32 mice. (B) The area of scrotal hernia was measured from 3- to 26-wk-old mice. Two-tailed Student’s t test, **P < 0.01 for Aromhum vs. WT, n = 32 mice. (C) H&E and (D) Masson’s trichrome staining of UAM and LAM from 26-wk-old WT and Aromhum mice. n = 10 mice for C and D. Yellow arrows in C and D indicate one of many atrophied myocytes. (Scale bars, 100 μm; Magnification: Inset in C, 40×.) Quantification of the percentage of connective tissue or fibrotic area (E) or myofiber area (F) in WT and Aromhum mice. Ten representative high-power fields were analyzed in each tissue. Two-way ANOVA with Tukey’s multiple comparison test, *P < 0.05, **P < 0.01, n = 8 mice per group.
Because inguinal hernia is associated with fibrosis of the LAM tissue, we analyzed the expression and promoter usage of aromatase and estrogen formation in the abdominal muscle tissue of Aromhum mice. We demonstrated that human aromatase mRNA but not mouse aromatase mRNA was readily detectable in LAM tissue, upper abdominal muscle (UAM) tissue, and quadriceps muscle (QM) tissue of Aromhum mice, whereas both human and mouse aromatase mRNA was absent in these muscle tissues in WT mice (Fig. 1D). 5′-RACE showed that aromatase mRNA was transcribed primarily from promoter I.4 of the human aromatase gene in abdominal muscle and QM tissues of Aromhum mice (SI Appendix, Table S1). Additionally, exon-specific RT-PCR showed that human aromatase expression was driven primarily by promoters I.4 and to a lesser extent PII in abdominal muscle tissues of Aromhum mice (Fig. 1E). The aromatase mRNA expression profile and promoter usage in other tissues of male Aromhum mice are summarized in SI Appendix, Table S1. Thus, human aromatase expression is driven by its native promoters in a wide variety of male Aromhum mouse tissues, including skeletal muscle (25, 35–37).
To determine whether humanized aromatase expression in the Aromhum mouse extragonadal tissue, including abdominal muscle, is accompanied by a change in tissue estrogen levels and circulating hormone levels, we measured tissue concentrations of estrogens [estrone (E1), E2, and estriol (E3)] using liquid chromatography-tandem mass spectrometry (LC-MS2) (Fig. 1F), and also compared peripheral serum levels of E2 and the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) using LC-MS2 and radioimmunoassay (SI Appendix, Fig. S1). Compared with WT mice, total estrogen levels in LAM tissues were significantly higher by 2.6-fold in Aromhum mice. Most importantly, LAM tissue levels of the biologically potent estrogen, E2, were 3.1-fold higher in Aromhum mice compared with WT littermates (Fig. 1F). However, serum E2 levels measured by LC-MS2 were not significantly different between WT and Aromhum males (Fig. 3C and SI Appendix, Fig. S1A). Serum FSH concentrations were significantly lower in Aromhum mice (64%) compared with WT mice. Decreased FSH levels in the Aromhum mice may be due to increased E2 production via human aromatase expression in hypothalamic tissue (SI Appendix, Table S1). Serum LH concentrations were lower but did not reach significance in Aromhum mice compared with WT littermates. These results show that local LAM tissue E2 levels, but not circulating E2 levels, led to hernia formation in male Aromhum mice.
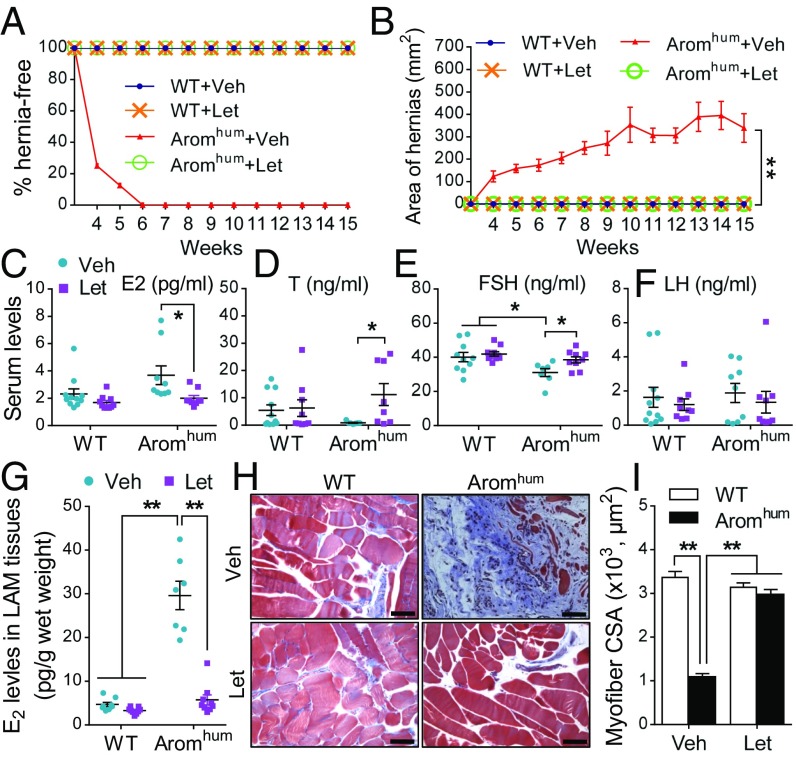
Fig. 3.
Aromatase inhibitor rescues LAM tissue fibrosis and myocyte atrophy and prevents the development of scrotal hernias in Aromhum mice. Three-week-old mice were treated with vehicle (Veh) or letrozole (Let) for 12 wk. (A) The incidence of scrotal hernia in Aromhum mice. (B) The area of scrotal hernia in Aromhum mice. Two-tailed Student’s t test, **P < 0.01. Serum E2 (C), T (D), FSH (E), and LH (F) were measured in WT and Aromhum mice after Veh or Let treatment. Statistical analysis by two-way ANOVA with Sidak’s multiple comparison test, *P < 0.05. (G) E2 levels in LAM tissue. Statistical analysis by two-way ANOVA with Tukey’s multiple comparison test, **P < 0.01. Serum and LAM E2 levels (C and G) were measured by LC-MS2 assay. (H) Representative photomicrographs of Masson’s trichrome-stained LAM sections of WT and Aromhum mice. (Scale bars, 100 μm.) (I) Quantification of myofiber cross-sectional area (CSA) in WT and Aromhum mice. A minimum of 1,000 cells in 10 different high-power fields were analyzed in each tissue. Two-way ANOVA with Tukey’s multiple comparison test, **P < 0.01. n = 10 mice per group.
The Development of Scrotal Hernias Is Associated with Fibrosis and Myocyte Atrophy in LAM Tissue of Aromhum Mice.
We studied WT and Aromhum mice from 1 to 26 wk of age for the development of hernias. Minimal lower abdominal bulging was first observed in 75% of Aromhum mice at 4 wk, with noticeable bulging by 8 wk in all Aromhum mice, followed by frank scrotal herniation by 12 wk in all Aromhum mice (Fig. 2A). Hernia size continued to increase during the whole observation period (up to 26 wk of age) (Fig. 2B).
We also studied the effects of muscle aromatase expression and estrogen production on skeletal muscle histology. UAM and QM did not show any major structural differences between WT and the Aromhum mice (Fig. 2C and SI Appendix, Fig. S4), while the morphology of extended LAM tissue that comprises the major part of hernia wall was markedly altered in Aromhum mice (Fig. 1 B and C). At 24 wk, LAM tissue of Aromhum mice showed a marked decrease in myocyte size and centralized nuclei, indicating myocyte atrophy (Fig. 2C, yellow arrow, Inset). Masson’s trichrome staining of LAM sections showed a marked increase in collagen deposition in the context of myocyte atrophy in Aromhum mice compared with WT mice (Fig. 2 D–F). Together, these results clearly indicate that hernia formation induced by LAM E2 production is associated with LAM tissue fibrosis and myocyte atrophy.
Administration of an Aromatase Inhibitor Prevents Scrotal Hernia Development in Aromhum Mice.
Aromatase inhibitors represent the most effective endocrine treatment for postmenopausal breast cancer (34, 38). The role of aromatase inhibitors in prevention of scrotal hernias, however, is unknown. Here we investigated whether blockage of aromatase activity using a slow-release aromatase inhibitor pellet (letrozole, 10 µg/d per mouse) can prevent scrotal hernia development in Aromhum mice. Intriguingly, none of the Aromhum mice administered subcutaneous letrozole at 3 wk of age developed scrotal hernia until the end of treatment (15 wk of age) (Fig. 3 A and B). Serum E2 levels were not significantly different between WT and Aromhum mice, but they significantly decreased in Aromhum mice after letrozole treatment (Fig. 3C). T levels in vehicle-treated Aromhum mice were lower compared with WT mice and were increased by levels comparable to those in WT mice after letrozole treatment (Fig. 3D). Similarly, FSH levels in vehicle-treated Aromhum mice were significantly lower compared with WT mice and increased to normal (WT) levels with letrozole treatment (Fig. 3E). However, letrozole treatment did not change serum LH levels in either WT or Aromhum mice, nor did it change serum E2, T, or FSH levels in WT mice (Fig. 3 C–F). Most importantly, LAM tissue E2 levels were 6.3-fold higher in Aromhum mice compared with WT littermates and letrozole treatment completely restored LAM E2 levels to normal (WT) levels (Fig. 3G). Development of fibrosis in the LAM tissue of Aromhum mice was completely prevented with letrozole treatment (Fig. 3H). The myofiber cross-sectional area in untreated Aromhum mice was significantly smaller than in WT mice, indicating muscle atrophy. Letrozole treatment completely prevented myofiber atrophy in Aromhum mice (Fig. 3 H and I). These data demonstrate that aromatase inhibition via letrozole treatment can inhibit LAM aromatase activity, restore LAM tissue E2 levels and serum T and FSH levels to normal levels, and prevent LAM tissue fibrosis, muscle atrophy, and hernia formation.
Higher Estrogen Sensitivity in LAM Tissue Is Accounted for by High ERα Levels in Stromal Fibroblasts.
E2 exerts its biological effects by binding and signaling through at least three distinct receptors, ERα, ERβ, and Gpr30 (39–41). The levels of ERα and ERβ in different muscle groups are markedly different (42). In Aromhum mice, ERβ mRNA levels were significantly lower in LAM tissue compared with UAM tissue (SI Appendix, Fig. S2A). Moreover, ERα mRNA levels were 350- and 597-fold higher than ERβ mRNA levels in UAM tissue and 610- and 1,786-fold higher than ERβ mRNA levels in LAM tissue of WT and Aromhum mice, respectively (SI Appendix, Fig. S2B). Additionally, although Gpr30 mRNA levels were significantly higher in LAM tissues than UAM tissues, ERα mRNA levels were 510- and 617-fold higher than Gpr30 mRNA levels in UAM tissue and 263- and 244-fold higher than Gpr30 mRNA levels in LAM tissue of WT and Aromhum mice, respectively (SI Appendix, Fig. S2 C and D). Most importantly, Gpr30 mRNA levels were significantly lower in LAM fibroblasts compared with UAM fibroblasts (SI Appendix, Fig. S2E). ERα mRNA levels were 47- and 54-fold higher than Gpr30 mRNA levels in UAM fibroblasts and 277- and 215-fold higher than Gpr30 mRNA levels in LAM fibroblasts of WT and Aromhum mice, respectively (SI Appendix, Fig. S2F). Thus, ERα seems to be the predominant receptor that plays a key role in estrogen sensitivity and hernia formation in LAM tissue of Aromhum mice. Furthermore, in muscle tissue, ERα mRNA levels were highest in LAM, modest in UAM, and lowest in QM in both WT and Aromhum mice (Fig. 4A). ERα mRNA levels in LAM of all mice were significantly higher than those in UAM. QM ERα mRNA levels were significantly lower than UAM and LAM in all mice. ERα protein levels tended to be higher but did not reach significance in LAM homogenates compared with UAM homogenates in either WT or Aromhum mice (SI Appendix, Fig. S3).
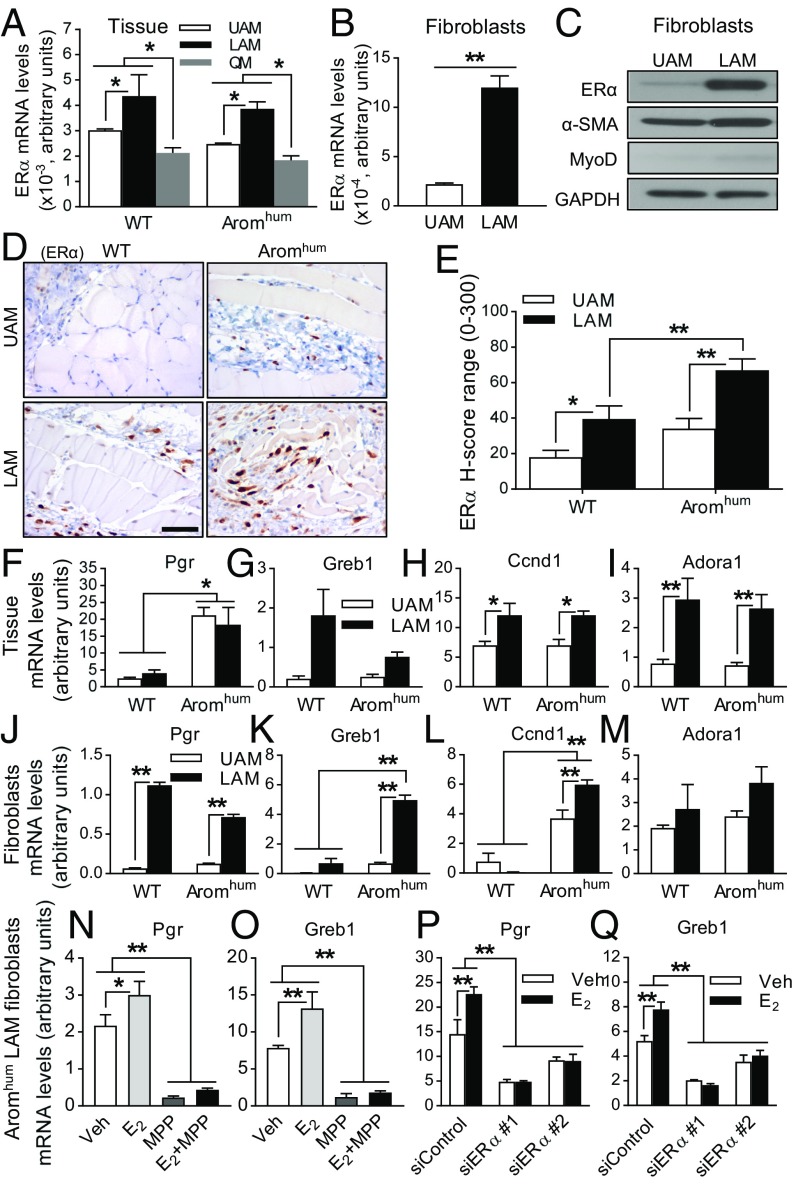
Fig. 4.
ERα expression is higher in LAM tissue fibroblasts of both WT and Aromhum, which contributes to the higher estrogen-responsive gene expression in LAM tissue of Aromhum mice. (A) Relative mRNA levels of ERα in UAM, LAM, and QM of WT and Aromhum mice. Two-way ANOVA with Tukey’s multiple comparison test. *P < 0.05. n = 10 mice in each group. (B) ERα mRNA levels and (C) ERα protein levels in primary fibroblasts from UAM and LAM of WT mice. The data shown in C are representative of three independent experiments. Two-tailed Student’s t test, **P < 0.01, n = 3. (D) ERα localization in UAM and LAM of WT and Aromhum mice was measured by IHC staining. (Scale bar, 50 μm.) (E) A minimum of 1,000 nuclei were counted in sections to calculate the average H-score of ERα in fibroblasts of UAM and LAM from WT and Aromhum mice. Two-way ANOVA with Sidek’s multiple comparison test, *P < 0.05, **P < 0.01, n = 10. mRNA levels of Pgr, Greb1, Ccnd1, and Adora1 in tissue lysates (F, G, H, and I; n = 9) and primary fibroblasts (J, K, L, and M; n = 6) of UAM and LAM from WT and Aromhum mice. mRNA levels of Pgr and Greb1 in LAM primary fibroblasts from Aromhum mice after MPP (10 µM) treatment (N and O) or siRNA-mediated knockdown of ERα (P and Q) in the presence or absence of E2 (10 nM). Cells were pretreated with MPP for 2 h before the addition of E2. Veh, vehicle. Two-way ANOVA with Sidak’s multiple comparison test, *P < 0.05, **P < 0.01. GAPDH mRNA or protein levels served as controls.
Because muscle is a heterogeneous tissue consisting of myocytes, stromal cells (fibroblasts), vascular endothelial cells, and other cell types, it is possible that specific expression of ERα in a particular cell type may not be accurately assessed by analyzing whole-tissue homogenates. Thus, we isolated fibroblasts from UAM and LAM tissues of WT mice and found that mRNA and protein levels of ERα were markedly higher in LAM fibroblasts than UAM fibroblasts (Fig. 4 B and C). Primary fibroblast specificity was confirmed by the presence of a fibroblast marker (α-SMA) and the absence of a myocyte marker (MyoD). We used immunohistochemistry (IHC) to further confirm ERα protein levels in LAM fibroblasts of WT and Aromhum mice. ERα immunoreactivity was almost exclusively observed in the stromal fibroblasts, but rarely present in myocytes (Fig. 4D and SI Appendix, Fig. S4). The H-score of ERα+ stromal cells was significantly higher in LAM tissue than in UAM tissue in both WT and Aromhum mice, and furthermore, it was markedly higher in Aromhum LAM tissue than in WT LAM tissue (Fig. 4E). The stromal component in QM had negligible ERα expression (SI Appendix, Fig. S5). Therefore, locally produced E2 in LAM tissues seems to be mediated via high LAM fibroblastic ERα expression, resulting in hernia formation in Aromhum mice.
ERα-Target Gene Expression Induced by Local E2 Excess in LAM Tissue Is Increased in Aromhum Mice.
To identify the early molecular events responsible for estrogen-induced muscle tissue fibrosis, myocyte atrophy, and hernia formation, we performed an RNA expression microarray analysis of LAM in WT and Aromhum mice at the age of 3 wk (before the start of morphologic changes in the muscle or hernia development). Multidimensional scaling analysis of the datasets from Aromhum and WT mice showed statistically significant differences; 92 genes were expressed preferentially in LAM of Aromhum mice and 33 genes were preferentially expressed in WT mice (Table 1 and Dataset S1). Pathway analysis listed the hepatic fibrosis pathway as the first up-regulated canonical pathway, and E2 was listed as the third upstream regulator in Aromhum mice (Tables 2 and 3). The expression of the previously established estrogen-target gene Greb1 was significantly higher (1.89-fold) in Aromhum mice than in WT mice. We verified the mRNA levels of Greb1 and several other estrogen-target genes (Pgr, Ccnd1, and Adora1) by real-time PCR in tissue lysates and primary fibroblasts of UAM and LAM from 3-wk-old WT and Aromhum mice (43–46). In general, mRNA levels of these estrogen-responsive genes tend to be higher in abdominal muscle tissues or fibroblasts with higher E2 levels (Aromhum vs. WT mice) and with higher ERα expression (LAM vs. UAM) (Fig. 4 F–M). Furthermore, to investigate whether ERα mediates the effects of E2 in LAM fibroblasts, we treated LAM primary fibroblasts from WT or Aromhum mice with E2 in the presence or absence of the E2/ER antagonist ICI 182780 or the ERα-selective E2 antagonist methyl-piperidino-pyrazole (MPP). E2 increased mRNA levels of Pgr and Greb1 after 48-h of treatment. ICI 182780 or MPP inhibited the stimulatory effect of E2 on gene expression of Pgr or Greb1 (Fig. 4 N and O and SI Appendix, Fig. S7 A, B, E, and F). ERα knockdown significantly down-regulated mRNA levels of Pgr and Greb1, which could not be restored with E2 treatment (Fig. 4 P and Q and SI Appendix, Figs. S6 and S7 I and J). These results strongly suggest that estrogen action in LAM fibroblasts of Aromhum mice was drastically enhanced via locally produced estrogen and high ERα expression, leading to hernia formation.
Table 1.
Microarray analysis of LAM tissue in WT and Aromhum mice
| Gene symbol | Definition | Fold-change | Adjusted P value |
| Top 13 up-regulated fibrotic genes in LAM of Aromhum mouse | |||
| Kiss1 | KiSS-1 metastasis-suppressor | 31.13 | 0.009 |
| Ren1 | Renin 1 structural | 7.87 | 0.026 |
| Emb | Embigin | 5.20 | 0.020 |
| Krt8 | Keratin 8 | 4.38 | 0.005 |
| Timp1 | Tissue inhibitor of metalloproteinase 1, transcript variant 2 | 4.19 | 0.009 |
| Krt7 | Keratin 7 | 3.05 | 0.007 |
| Spon2 | Spondin 2, extracellular matrix protein | 2.83 | 0.058 |
| Krt18 | Keratin 18 | 2.71 | 0.013 |
| Spon1 | Spondin 1, (f-spondin) extracellular matrix protein | 2.54 | 0.009 |
| Tnc | Tenascin C | 2.52 | 0.079 |
| Plod2 | Procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 | 2.42 | 0.018 |
| Eln | Elastin | 2.38 | 0.020 |
| Col8a1 | Collagen, type VIII, α1 | 2.21 | 0.040 |
| Top 8 down-regulated androgen responsive genes in LAM of Aromhum mouse | |||
| Amd2 | S-adenosylmethionine decarboxylase 2 | 3.43 | 0.036 |
| Cyp2e1 | Cytochrome P450, family 2, subfamily e, polypeptide 1 | 3.22 | 0.045 |
| Cpeb4 | Cytoplasmic polyadenylation element binding protein 4 | 1.89 | 0.025 |
| Idh3g | Isocitrate dehydrogenase 3 (NAD+), γ | 1.85 | 0.015 |
| Tiam1 | T cell lymphoma invasion and metastasis 1 | 1.77 | 0.037 |
| Cntnap2 | Contactin associated protein-like 2 | 1.75 | 0.043 |
| Tst | Thiosulfate sulfurtransferase, mitochondrial | 1.75 | 0.037 |
| Hrasls | HRAS-like suppressor | 1.73 | 0.043 |
Fibrotic genes were up-regulated and androgen-responsive genes were down-regulated in Aromhum mice vs. WT mice.
Table 2.
Ingenuity pathway analysis revealed top three up-regulated canonical pathways in Aromhum mice
| Top three up-regulated canonical pathways | P value |
| Hepatic fibrosis/hepatic stellate cell activation | 1.18E-04 |
| Agranulocyte adhesion and diapedesis | 1.18E-03 |
| Parkinson’s signaling | 1.99E-03 |
Table 3.
Ingenuity pathway analysis revealed top three upstream regulators in Aromhum mice
| Top three upstream regulators | P value |
| SOX4 | 3.33E-08 |
| SRF | 5.39E-07 |
| E2 | 2.36E-06 |
The Role of Fibroblast Proliferation and Excessive ECM Formation in Estrogen-Induced Hernia Formation.
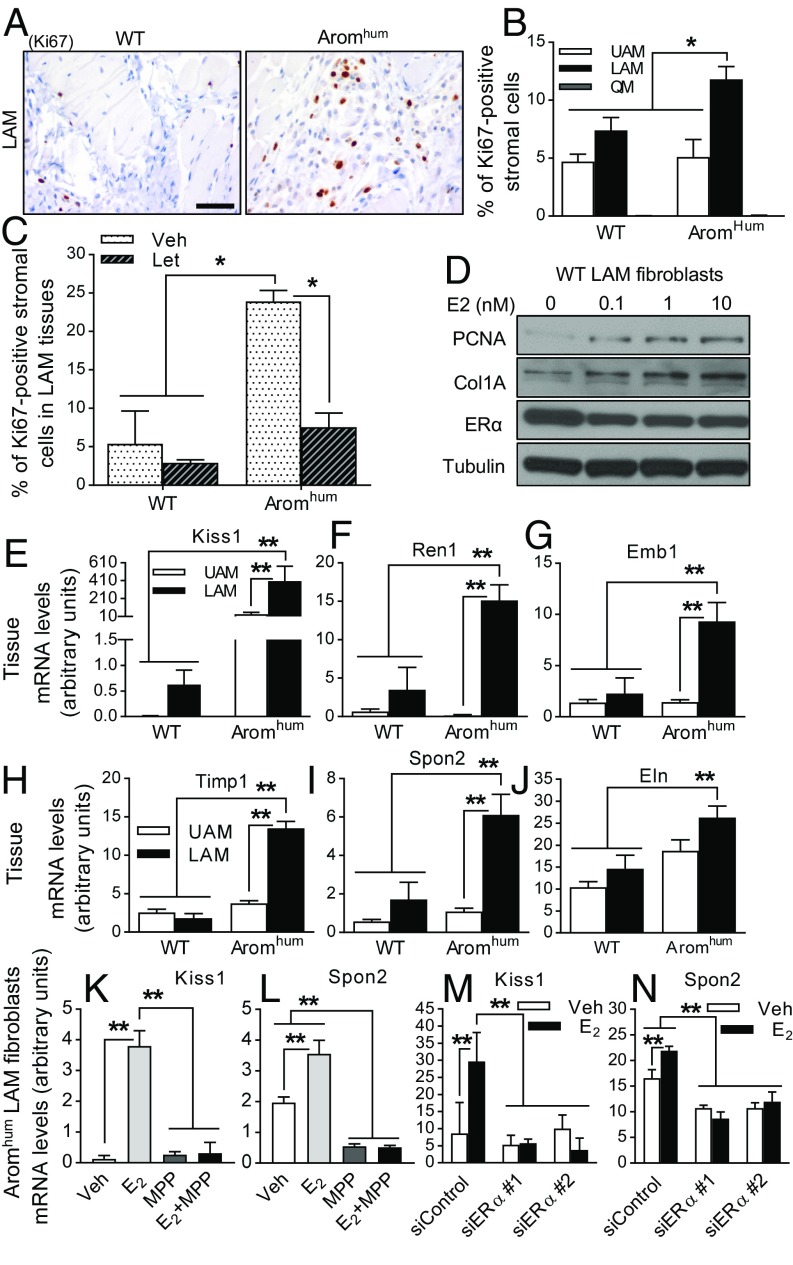
Fibroblasts constitute the key cell type of the stroma and are important for wound healing and collagen formation in fibrotic tissues (47). To further determine the underlying mechanisms of locally produced estrogen in fibroblast proliferation, function, and hernia formation in LAM, Ki67 immunostaining (indicating cell proliferation) was performed in UAM, LAM, and QM of WT and Aromhum mice. Ki67 immunoreactivity was predominantly observed in the LAM stromal component and scarcely present in myocytes of WT and Aromhum mice. Ki67 immunostaining, although low, could be detected in UAM stromal tissue and was negligible in the QM stromal component of both mice. The highest percentage of Ki67+ nuclei was observed in LAM stromal cells of Aromhum mice (Fig. 5 A and B). Letrozole treatment strikingly decreased the number of Ki67+ fibroblasts in LAM tissue of Aromhum mice (Fig. 5C). These results show that estrogen induced-fibroblast proliferation was increased in vivo in LAM tissue of Aromhum mice.
Fig. 5.
Increased ERα expression and estrogen production in LAM tissue are responsible for LAM fibroblast proliferation and collagen formation in Aromhum mice. (A) Cell proliferation indicated by Ki67 immunoreactivity was measured in LAM tissue. (Scale bar, 50 μm.) (B) A minimum of 1,000 nuclei were counted in sections to calculate the percent of Ki67+ stromal cells in UAM, LAM, and QM of WT and Aromhum mice. Two-way ANOVA with Sidak’s multiple comparison test, *P < 0.05, n = 10. (C) The effect of letrozole (Let) treatment on stromal cell proliferation determined by calculation of the percent of Ki67+ nuclei in UAM and LAM of WT and Aromhum mice treated with letrozole. Veh, vehicle. Two-way ANOVA with Tukey’s multiple comparison test, *P < 0.05, n = 8–11. (D) Cell proliferation marker proliferating cell nuclear antigen (PCNA) and protein levels of type I collagen (Col1A) were determined by immunoblotting in primary LAM fibroblasts of WT mice treated with the physiological doses of E2 for 24 h. Tubulin protein levels served as the loading control. Data are representative of three independent experiments. mRNA levels of profibrotic genes Kiss1 (E), Ren1 (F), Emb (G), Timp1 (H), Spon2 (I), and Eln (J) were measured in UAM and LAM tissues of WT and Aromhum mice at 3 wk of age. mRNA levels of Kiss1 and Spon2 in primary LAM fibroblasts from Aromhum mice after MPP (10 µM) treatment (K and L) or siRNA-mediated knockdown of ERα (M and N) in the presence or absence of E2 (10 nM). Cells were pretreated with MPP for 2 h before the addition of E2. Veh, vehicle. GAPDH mRNA levels served as the control. Two-way ANOVA with Tukey’s multiple comparison test, *P < 0.05, **P < 0.01, n = 10 mice in each group.
Primary fibroblasts isolated from LAM tissue of WT mice were treated with physiological doses of E2 (0.1 nM to 10 nM) for 24 h; cell proliferation, indicated by proliferating cell nuclear antigen levels, and fibrosis, indicated by Col1A, were increased in proportion to the dose of estrogen (Fig. 5D). ERα protein levels were decreased after E2 treatment, suggesting the effectiveness of the E2 treatment. Interestingly, RNA microarray analysis of LAM of Aromhum mice demonstrated that of the top 33 up-regulated genes, 13 are known to be involved in the fibrotic response (Table 1). The higher expression of the fibrosis-related genes Kiss1, Ren1, Emb, Timp1, Spon2, and Eln in LAM of Aromhum compared with WT tissues was verified by real-time PCR (Fig. 5 E–J). In Aromhum mice, mRNA levels of Kiss1, Ren1, Emb, Timp1, and Spon2 in LAM were also significantly higher compared with UAM (Fig. 5 E–I). Microarray analysis showed that the fibrotic pathways were activated in LAM tissue of Aromhum mice (Table 2). Furthermore, we treated LAM primary fibroblasts from WT or Aromhum mice with E2 in the presence or absence of the E2/ER antagonist ICI 182780 or the ERα-selective E2 antagonist MPP. E2 increased mRNA levels of Kiss1 and Spon2 after a 48-h treatment. ICI 182780 or MPP inhibited the stimulatory effect of E2 on these two fibrotic gene expressions (Fig. 5 K and L and SI Appendix, Fig. S7 C, D, G, and H). ERα knockdown significantly down-regulated mRNA levels of Kiss1 and Spon2 in the presence of E2 treatment (Fig. 5 M and N and SI Appendix, Figs. S6 and S7 K and L). Together, these results demonstrated that E2 excess and higher ERα expression induced not only fibroblast proliferation but also a sharp increase in ECM formation in LAM fibroblasts of Aromhum mice.
Aromhum Mice also Display Decreased Circulating T Levels and Androgen-Responsive Gene Expression.
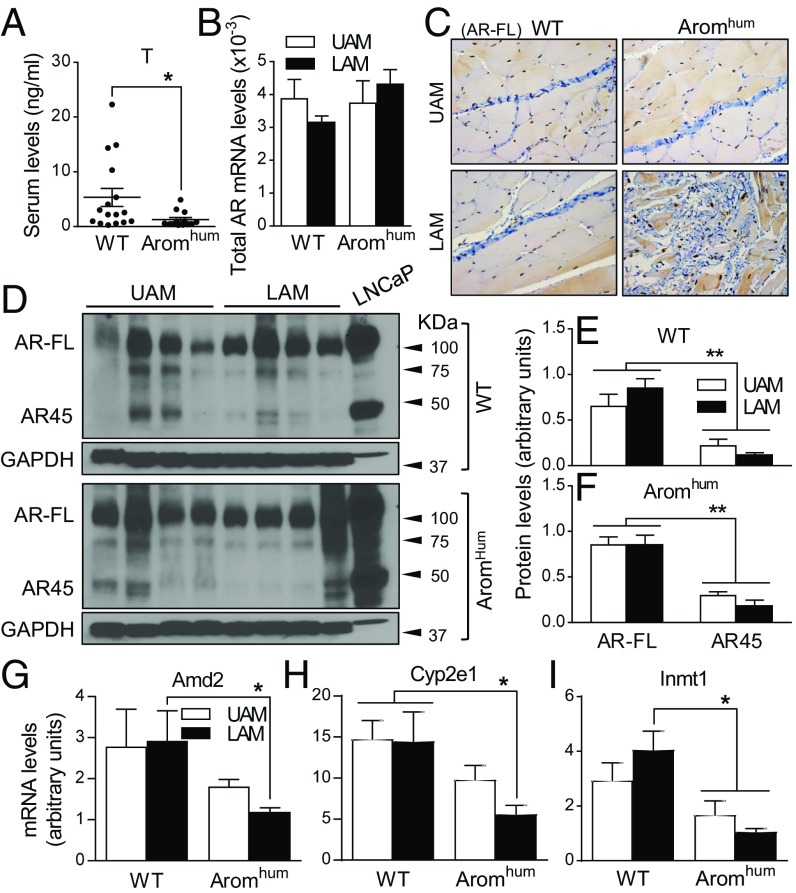
Intriguingly, we found that circulating T levels were significantly lower in Aromhum mice than in WT controls (Fig. 6A) and were restored to levels comparable to those in WT mice after letrozole treatment (Fig. 3D). These data suggest that elevated brain aromatase and estrogen formation in Aromhum mice may suppress gonadotropins, leading to decreased testicular T secretion. An alternative interpretation could be that increased aromatase activity leads to T depletion via converting it into E2. This, however, is unlikely because the roughly estimated rate of conversion of serum T to E2 is about 0.3% in Aromhum mice (48). T exerts its biological action via binding to the AR. Besides the full-length AR (AR-FL), mRNA levels of AR45, one of the AR variants (49), were also reported to be present in skeletal muscle tissue (50, 51). Using primers that amplify total AR (both AR-FL and AR45) in UAM and LAM tissues, we found similar levels of total AR mRNA in WT and Aromhum mice (Fig. 6B). Using an antibody that specifically recognizes only AR-FL, AR-FL immunostaining was observed in both myocytes and stromal cells (Fig. 6C). Interestingly, using a second antibody recognizing both AR-FL and AR45 in mouse and human for immunoblotting, both AR-FL and AR45 proteins were identified in abdominal skeletal muscle tissues (Fig. 6D). Human prostate cancer cell line LNCaP expressed both AR-FL and AR45 and served as positive controls (50, 52, 53). AR-FL protein levels were significantly higher than AR45 protein levels in all UAM and LAM tissues of both WT and Aromhum mice (Fig. 6 D–F). However, protein levels of AR-FL and AR45 did not significantly vary between LAM and UAM in WT or Aromhum mice (Fig. 6 D–F). Additionally, AR-FL and AR45 protein levels were not different between WT UAM and Aromhum UAM, nor were they different between WT LAM and Aromhum LAM (SI Appendix, Fig. S8). Overall, AR-FL seemed to be the predominant AR type in all LAM and UAM tissues examined.
Fig. 6.
Androgen action is lower in LAM of Aromhum mice. (A) Serum T levels in WT and Aromhum mice were measured by radioimmunoassay. Mouse sera were collected from 26-wk-old mice. Two-tailed Student’s t test, *P < 0.05, n = 15. (B) Total AR mRNA levels (AR-FL and AR45) in UAM and LAM tissues of WT and Aromhum mice. Two-way ANOVA with Tukey’s multiple comparison test, n = 10. (C) Nuclear AR-FL assessed by IHC in UAM and LAM of WT and Aromhum mice. n = 8. (Scale bar, 50 μm; Magnification: C, 40×.) (D) AR-FL and AR45 protein levels in UAM and LAM tissues of WT and Aromhum mice. GAPDH protein levels served as the loading control. Human prostate cancer cell line LNCaP served as AR-FL and AR45 positive controls. Quantification of AR-FL and AR45 by densitometry in UAM and LAM of WT (E) and Aromhum (F) mice. Two-way ANOVA with Sidak’s multiple comparison test, **P < 0.01, n = 4 mice in each group. mRNA levels of androgen-responsive genes Amd2 (G), Cyp2e1(H), and Inmt1 (I) in UAM and LAM tissues of WT and Aromhum mice. GAPDH mRNA levels served as the control. Two-way ANOVA with Tukey’s multiple comparison test, *P < 0.05, n = 10 mice per group.
Microarray analysis using LAM tissues of 3-wk-old WT and Aromhum mice showed that 8 of the 33 genes that were significantly down-regulated in LAM of Aromhum mice encode androgen-response proteins (Table 1 and Dataset S1). Real-time PCR verified that mRNA levels of the androgen target genes Amd2, Cyp2e1, and Inmt were significantly lower in LAM of Aromhum mice compared with LAM of WT mice (Fig. 6 G–I). In addition to higher estrogenic activity, lower androgen action in LAM tissue via low serum T levels may also contribute to hernia formation in Aromhum mice.
Men with Inguinal Hernia Show Higher ERα Expression, Proliferative Activity, and ECM Formation in Stroma and Lower AR Expression in Myocytes in LAM Tissues.
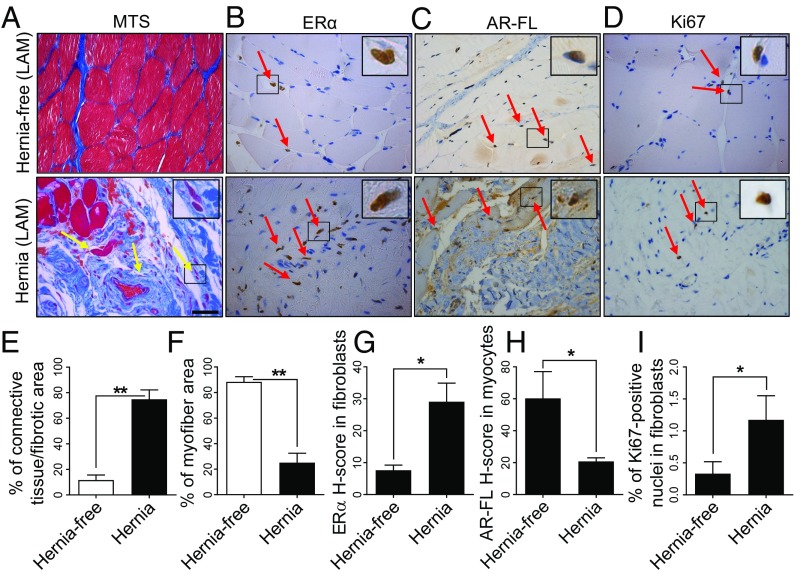
To determine whether the patterns of ERα and AR protein expression, stromal proliferation, and ECM formation in men are comparable to those in mice, IHC for ERα, AR (using an antibody that specifically recognizes AR-FL), and Ki67 and Masson’s trichrome staining (to detect fibrosis) were performed in normal LAM tissue from hernia-free men (n = 6; age 50–68 y) or extended LAM tissue from men with indirect inguinal hernia (n = 6; age 60–77 y). Masson’s trichrome staining indicated higher ECM formation and numbers of fibroblasts with myofiber atrophy in LAM of hernia patients compared with hernia-free patients (Fig. 7 A, E, and F). Similar to the mouse muscle tissue ERα expression pattern, human ERα expression was significantly higher in the stromal cells of LAM tissue from hernia patients (H-score: 29.06 ± 5.86) compared with tissues from hernia-free patients (H-score: 7.64 ± 1.59). ERα expression was negligible in myocytes (Fig. 7 B and G). AR-FL expression in LAM myocytes was significantly lower in hernia patients (H-score: 20.88 ± 2.17) than in hernia-free patients (H-score: 59.16 ± 17.39) (Fig. 7 C and H). Stromal cell proliferation indicated by Ki67 staining was significantly increased in hernia patients (1.2%) compared with hernia-free controls (0.3%) (Fig. 7 D and I). Thus, cell-specific ERα expression pattern, cell proliferation, and fibrosis in human indirect inguinal hernia tissue are similar to those observed in the Aromhum mouse hernia model.
Fig. 7.
(A) Masson’s trichrome staining (MTS), (B) immunostaining for ERα, (C) AR-FL, and (D) Ki67 in LAM tissue from hernia-free and hernia patients. Yellow arrows indicate atrophic myocytes. Red arrows indicate brown positive staining. (Scale bar, 50 μm; Magnification: Insets in A–D, 40×.) Quantification of the percentage of connective tissue or fibrotic area (E) and myofiber area (F), the average H-score (range 0–300) for ERα in fibroblasts (G) and AR-FL in myocytes (H), and the percentage of Ki67+ nuclei (I) in fibroblasts from hernia-free and hernia patients. Two-tailed Student’s t test, *P < 0.05, **P < 0.01, n = 6 per group.
Discussion
Aromhum mice represent a unique and pathologically relevant experimental model to study the relationship between aromatization of androgen to estrogen, the downstream estrogenic and androgenic effects in muscle tissue, and LAM fibrosis and atrophy, leading to hernia development. Human aromatase expression driven by its alternatively used cognate promoters (I.4 and to a lesser extent PII) in Aromhum mice resembled the human patterns of age-related increase in aromatase expression and estrogen formation in peripheral tissues, including LAM tissue, and an accompanying decrease in circulating T levels (25). Our data strongly indicate that locally produced E2 acting on highly estrogen-sensitive and ERα-rich LAM fibroblasts led to stromal fibrosis, myocyte atrophy, and eventually inguinal hernia formation. The role of E2 in this phenomenon is clear because the inhibition of E2 production by an aromatase inhibitor prevented hernia formation. The possibly contributing roles of decreased T levels that are observed in Aromhum mice and restored to normal with an aromatase inhibitor, however, are less clear. Decreased T levels are possibly due to increased brain E2 levels via the brain expression of human aromatase, leading to decreased gonadotropin and then testicular T secretion. As the incidence of inguinal hernia and peripheral aromatase expression increase with age in men, our findings have a particular clinical significance not only for understanding the mechanisms underlying maintenance of skeletal muscle mass in various body sites, but also for assessing hernia risk and developing strategies for hernia prevention in a subset of elderly men (27–29).
Previous studies showed that postnatal systemic administration of exogenous estrogen or ubiquitous overexpression of a full-length aromatase cDNA with strikingly high circulating estrogen levels led to the formation of scrotal hernias in mice; in utero estrogen exposure was not necessary for this phenotype (9, 54). Our humanized aromatase mouse model is unique in that the estrogenic effect on LAM tissue and hernia formation are primarily mediated via local estrogen production by aromatase activity from the human CYP19A1 (aromatase) gene expressed in the skeletal muscle tissue, with normal circulating E2 levels. This model of muscle atrophy and hernia development is therefore more physiologically relevant to these common pathologies observed in a subset of elderly men.
Estrogen exerts its physiological functions by binding to its receptors ERα, ERβ, and GPR30 (39–41). Studies of ER knockout mice show that ERα is primarily involved in the classic actions of estrogens (i.e., sexual differentiation, fertility, uterine function, and lactation) (39, 40). ERβ has been shown to play biological roles in the central nervous system, the immune system, the ovary, and the prostate (55, 56). GPR30, on the other hand, has been linked to certain physiological and pathological effects regulated by estrogen on the central nervous, immune, renal, reproductive, and cardiovascular systems (41, 57–59). Because ERβ and Gpr30 mRNA levels in LAM or UAM tissues are either barely detectable or extremely lower than ERα in our hands, the estrogenic effects on LAM fibrosis and hernia are most likely mediated primarily by ERα signaling. Indeed, we found that ERα mRNA and protein were predominantly present in the prominent perimuscular stromal fibroblast compartment of LAM tissue in both WT and Aromhum mice. Additionally, ERα levels in LAM tissue fibroblasts were higher than those in UAM or QM tissues. Moreover, ICI 182780 (which opposes E2 action via degradation of the ER), MPP (which is an ERα-selective E2 antagonist), or ERα knockdown diminished estrogenic gene expression in LAM fibroblasts. Thus, we conclude that locally produced estrogen in Aromhum LAM tissue is mediated via fibroblastic ERα, giving rise to increased stromal cell proliferation, fibrosis, muscular atrophy, and hernia development. This explains, at least in part, why atrophy and fibrosis develop in LAM tissue of Aromhum mice, but not in other muscle groups (i.e., UAM and QM).
Gene-expression profiling allowed us to identify a number of molecular pathways and target genes activated early by E2/ERα in LAM tissues before the hernias become manifest. These pathways include ERα-driven fibroblast activation and fibrosis pathways (44–46, 60, 61). Consistent with these findings, E2 is found to induce fibrosis in pathologic tissues, including gynecomastia, uterine fibroid tumors, and the skin of patients with systemic sclerosis (62–64). Several known E2/ERα target genes were highly expressed in LAM tissue of Aromhum mice. Moreover, some of these genes, (e.g., Greb1 and Pgr) were selectively induced in primary fibroblasts of LAM tissue, suggesting that these effects took place primarily in the fibroblast compartment of LAM tissue. Greb1 is a chromatin-bound ER coactivator and is essential for ER-mediated transcription (61). Greb1 is also one of the most highly estrogen-inducible genes and correlates well with changes in ER activity following breast cancer treatment (43, 65). Thus, Greb1 may also contribute to fibroblast proliferation in LAM. Moreover, the estrogen-responsive fibrotic genes, including Kiss1, Krt8, Krt7, Spon2, Krt18, Tnc, Plod2, and Eln, were also increased in LAM of Aromhum mice (Table 1), as has been reported for several other tissues (66–72). On the other hand, several other well-known estrogen response genes (e.g., Adora1, Tff1, and Susd3) were not induced by aromatase expression in LAM tissue (44, 45, 73), suggesting epigenetic differences between tissues or cell types may account for E2/ERα induction of a select group of genes.
T is the major substrate of the aromatase enzyme. T is not only converted by aromatase to E2 in target tissues for estrogenic action, but is also converted by 5α-reductatase-1 or -2 into a potent and nonaromatizable androgen, DHT (74). mRNA levels of 5α-reductase-1 or -2 were undetectable or barely detectable in LAM tissue. Thus, in LAM, androgen action must be provided primarily via an interaction of T with AR. In fact, in double 5α-reductase knockout mice (for both types 1 and 2), T was shown to exert androgenic action via AR (74).
T and AR exert anabolic effects on skeletal muscle, resulting in muscle protein synthesis and increased muscle mass (75, 76). Overall, AR-FL seems to be the predominant AR type in both LAM and UAM tissue, suggesting a more important role of AR-FL in both WT and Aromhum LAM tissues. The precise role of AR45 in abdominal muscle tissue, however, needs further investigation. We also found that serum T was significantly lower in Aromhum mice, which may contribute to decreased androgen action. These results indicate that low circulating T levels, together with local estrogen excess, shift the steroid balance from androgen to estrogen in Aromhum mice or in a subset of elderly men, leading to muscle atrophy and hernia, possibly via decreased muscle mass.
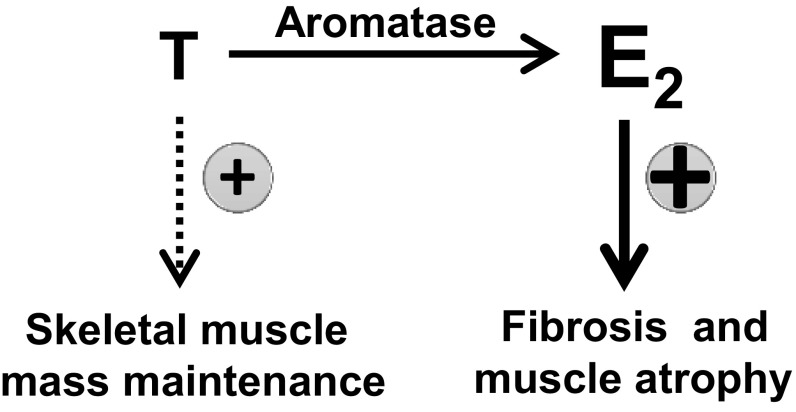
In summary, the lower portion of the LAM tissue adjacent to the scrotal opening is particularly enriched with ERα-expressing fibroblasts and thus sensitive to E2. Expression of aromatase in mice, which directly converts circulating T to E2 locally in the muscle or in the brain, leads to stromal proliferation, muscle atrophy, and hernia development in LAM tissue via up-regulation of estrogenic action and down-regulation of androgenic action (Fig. 8). All Aromhum mice uniformly developed fibrosis, myocyte atrophy, and hernia, which was entirely blocked and prevented by an aromatase inhibitor. There are currently no experimental or approved medical options for the prevention of inguinal hernias associated with skeletal muscle fibrosis and atrophy in a subset of men. In particular, the surgical repair of a recurrent hernia is quite problematic and carries a high risk of treatment failure or recurrence. Intriguingly, our findings uncover a previously unrecognized mechanism for LAM fibrosis and atrophy and inguinal hernia formation and open new horizons for drug development to prevent hernia, especially recurrent hernia after surgery in vulnerable populations, such as elderly men. Currently available aromatase inhibitors or analogs of androgen may provide alternative or complementary management modalities in addition to surgical repair.
Fig. 8.
Schematic demonstrating the effect of a shift from androgen to estrogen action induced by human aromatase gene expression in LAM tissue on fibrosis, myocyte atrophy, and hernia formation in mice.
Materials and Methods
Aromhum Mouse Maintenance, Hernia Assessment, and Letrozole Treatment.
The Aromhum mouse (FVB/N background) was generated and genotyped in our laboratory, as previously described (34). Aromhum transgenic mice contain the complete human aromatase coding region, a >75-kb promoter region including promoters I.4, I.7, I.f, I.6, I.3 and PII, and the 3′-polyadenylation site. Mice were maintained on a 14-h light:10-h dark cycle with standard chow (7912; Harlan Teklad) and water available ad libitum. All animal experiments were approved by and conducted in accordance with guidelines established by the Institutional Animal Care and Use Committee at Northwestern University. Animals were randomly used for all experiments in a blinded manner. Hernia development in 32 Aromhum male mice was monitored by weekly visual inspection and palpation from 3 to 26 wk of age. Age-matched WT male littermates were used as controls. Hernia dimensions were measured using a digital caliper, and hernia area was calculated by the formula, area (mm2) = length (mm) × width (mm). At the designated endpoints, UAM, LAM, and QM from each individual mouse were resected, with one-half of the tissue snap-frozen in liquid nitrogen, and the other half fixed in 4% phosphate-buffered paraformaldehyde for histological and IHC analyses. All tissue and serum samples were collected from mice between 10:00 AM and 12:00 PM (noon) to avoid possible variability in daily hormone fluctuations. In some experiments, male mice were randomly treated with letrozole (10 µg/d per mouse) using 90-d continuous-release pellets (Innovative Research of America) or control pellets starting at 3 wk of age (77). Pellets were implanted subcutaneously on the lateral side of the neck between the ear and the shoulder.
Human Subjects.
We recruited six hernia-free men (aged 50–68 y) and six men with hernias (aged 60–77 y) from the First Affiliated Hospital of Nanjing Medical University in China. Men were excluded if they had chronic debilitating disease, had undergone chemotherapy or radiation therapy, or had received any hormonal treatments within the past 3 mo. Muscle biopsy specimens from the inguinal area were obtained and preserved in 10% formalin and embedded in paraffin. The human study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanjing Medical University and complied strictly with the national ethical guidelines of China. Written informed consent was obtained from all participants before inclusion in the study. All participants were identified by assigned numbers.
Primary Mouse Skeletal Muscle Tissue Fibroblast Culture, Hormonal Treatments, and Small-Interfering RNA Knockdown.
Isolation and culture of primary skeletal muscle tissue fibroblasts were performed based on a modified version of an established protocol for culturing human or mouse adipose fibroblasts and mouse skeletal myoblasts (78–80). In brief, UAM and LAM tissues from six WT and six Aromhum mice were minced and digested with collagenase D (1.5 U/mL), dispase II (2.4 U/mL), and CaCl2 (2.5 mM) at 37 °C for 30–60 min. Single-cell suspensions were prepared by filtration through a 75-μm sieve. Fibroblasts in cell suspensions were allowed to attach collagen-coated Petri dishes for 15 min and unattached myoblasts were removed. Primary fibroblasts were obtained by growing cells in DMEM/F-12 medium with 10% FBS. Under such conditions, there was preferential growth of fibroblastic cells which comprised 99% of the total population within 2 wk (80). Cells were grown to 80% confluence and placed in serum-free and phenol-free medium for 16 h before treatment. Primary fibroblasts were incubated in serum-free and phenol-free DMEM/F-12 in the absence or presence of physiological doses of E2 (0.1 nM, 1 nM, and 10 nM; Sigma-Aldrich) for 24–48 h. Cells were pretreated with ICI 182780 (100 nM) or MPP (10 µM) for 2 h before the addition of E2. Cell extracts were prepared for real-time RT-PCR analysis or immunoblotting. To knock down endogenous ERα expression, primary fibroblasts were transfected with two separate ON-TARGETplus mouse ERα siRNAs (Dharmacon) using DharmaFECT 1 transfection reagent (Dharmacon) for 48 h. ON-TARGETplus nontargeting control siRNA (Dharmacon) was transfected as a negative control.
Data Availability.
The RNA microarray data that support the findings of this study have been deposited to the National Center for Biotechnology Information Gene Expression Omnibus database under the accession no. GSE92748 (https://www.ncbi.nlm.nih.gov/geo/).
Statistical Analysis.
Results are expressed as mean ± SEM, unless otherwise indicated. Statistically significant differences at P < 0.05 were determined using two-tailed Student’s t test, one-way ANOVA, or two-way ANOVA. All statistical tests were performed using the GraphPad Prism software.
Extended method and information about RNA isolation and quantitative real-time PCR, exon-specific RT-PCR amplification, histology, Masson’s trichrome staining, IHC, scoring of immunoreactivity, protein extraction and immunoblotting, serum and tissue hormone levels, and microarrays, and data analyses are described in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Elizabeth M. McNally, Dr. Pin Yin, Dr. Alexis R. Demonbreun, Dr. Matthew J. Schipma, and Dr. Matthew T. Dyson at Northwestern University for all their help and insight; the Ligand Assay & Analysis Core at the University of Virginia Center for Research in Reproduction for measuring serum sex steroid hormones and gonadotrophins; the Mouse Histology & Phenotyping Laboratory and the Pathology Core Facility Laboratories at Northwestern University for performing immunohistochemistry; and Dean Evans from Novartis for providing the aromatase inhibitor letrozole. This work was supported by the NIH Grant R37-HD36891 (to S.E.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE92748).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807765115/-/DCSupplemental.
References
- 1.Chung L, Norrie J, O’Dwyer PJ. Long-term follow-up of patients with a painless inguinal hernia from a randomized clinical trial. Br J Surg. 2011;98:596–599. doi: 10.1002/bjs.7355. [DOI] [PubMed] [Google Scholar]
- 2.Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83:1045–1051, v–vi. doi: 10.1016/S0039-6109(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 3.Matthews RD, Neumayer L. Inguinal hernia in the 21st century: An evidence-based review. Curr Probl Surg. 2008;45:261–312. doi: 10.1067/j.cpsurg.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Bay-Nielsen M, Perkins FM, Kehlet H. Danish Hernia Database Pain and functional impairment 1 year after inguinal herniorrhaphy: A nationwide questionnaire study. Ann Surg. 2001;233:1–7. doi: 10.1097/00000658-200101000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malangoni MA, Rosen MJ. Hernias. In: Townsend C, Beauchamp RD, Evers BM, Mattox K, editors. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 20th Ed. Saunders Elsevier; Philadelphia: 2008. pp. 1092–1119. [Google Scholar]
- 6.Matthews RD, et al. Veterans Affairs Cooperative 456 Studies Program Investigators Factors associated with postoperative complications and hernia recurrence for patients undergoing inguinal hernia repair: A report from the VA Cooperative Hernia Study Group. Am J Surg. 2007;194:611–617. doi: 10.1016/j.amjsurg.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Jiang ZP, Li YR, Zong Z, Chen S. Long-term outcome for open preperitoneal mesh repair of recurrent inguinal hernia. Int J Surg. 2015;19:134–136. doi: 10.1016/j.ijsu.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Gardner WU. Sexual dimorphism of the pelvis of the mouse, the effect of estrogenic hormones upon the pelvis and upon the development of scrotal hernias. Am J Anat. 1936;59:459–483. [Google Scholar]
- 9.Hazary S, Gardner WU. Influence of sex hormones on abdominal musculature and the formation of inguinal and scrotal hernias in mice. Anat Rec. 1960;136:437–443. doi: 10.1002/ar.1091360402. [DOI] [PubMed] [Google Scholar]
- 10.Bjorn JC, Gardner WU. Inguinal hernias in female mice treated with androgens or bearing grafts of testes. Endocrinology. 1956;59:48–54. doi: 10.1210/endo-59-1-48. [DOI] [PubMed] [Google Scholar]
- 11.Amato G, et al. Histological findings in direct inguinal hernia: Investigating the histological changes of the herniated groin looking forward to ascertain the pathogenesis of hernia disease. Hernia. 2013;17:757–763. doi: 10.1007/s10029-012-1032-0. [DOI] [PubMed] [Google Scholar]
- 12.Amato G, et al. Muscle degeneration in inguinal hernia specimens. Hernia. 2012;16:327–331. doi: 10.1007/s10029-011-0890-1. [DOI] [PubMed] [Google Scholar]
- 13.Amato G, et al. Histological findings of the internal inguinal ring in patients having indirect inguinal hernia. Hernia. 2009;13:259–262. doi: 10.1007/s10029-009-0483-4. [DOI] [PubMed] [Google Scholar]
- 14.Gunnarsson U, Degerman M, Davidsson A, Heuman R. Is elective hernia repair worthwhile in old patients? Eur J Surg. 1999;165:326–332. doi: 10.1080/110241599750006857. [DOI] [PubMed] [Google Scholar]
- 15.Kingsnorth A, LeBlanc K. Hernias: Inguinal and incisional. Lancet. 2003;362:1561–1571. doi: 10.1016/S0140-6736(03)14746-0. [DOI] [PubMed] [Google Scholar]
- 16.Zendejas B, et al. Incidence of inguinal hernia repairs in Olmsted County, MN: A population-based study. Ann Surg. 2013;257:520–526. doi: 10.1097/SLA.0b013e31826d41c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goh VH, Tong TY, Mok HP, Said B. Interactions among age, adiposity, bodyweight, lifestyle factors and sex steroid hormones in healthy Singaporean Chinese men. Asian J Androl. 2007;9:611–621. doi: 10.1111/j.1745-7262.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- 18.Jasuja GK, et al. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 2013;68:733–740. doi: 10.1093/gerona/gls216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JY, et al. Decline of serum levels of free testosterone in aging healthy Chinese men. Aging Male. 2005;8:203–206. doi: 10.1080/13685530500356010. [DOI] [PubMed] [Google Scholar]
- 20.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: Results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 21.Leifke E, et al. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: Cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 2000;53:689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 22.Ferrini RL, Barrett-Connor E. Sex hormones and age: A cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 23.Simon D, et al. The influence of aging on plasma sex hormones in men: The Telecom Study. Am J Epidemiol. 1992;135:783–791. doi: 10.1093/oxfordjournals.aje.a116365. [DOI] [PubMed] [Google Scholar]
- 24.Orwoll E, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91:1336–1344. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- 25.Larionov AA, et al. Aromatase in skeletal muscle. J Steroid Biochem Mol Biol. 2003;84:485–492. doi: 10.1016/s0960-0760(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 26.Matsumine H, Hirato K, Yanaihara T, Tamada T, Yoshida M. Aromatization by skeletal muscle. J Clin Endocrinol Metab. 1986;63:717–720. doi: 10.1210/jcem-63-3-717. [DOI] [PubMed] [Google Scholar]
- 27.Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994;78:428–432. doi: 10.1210/jcem.78.2.8106632. [DOI] [PubMed] [Google Scholar]
- 28.Cleland WH, Mendelson CR, Simpson ER. Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab. 1985;60:174–177. doi: 10.1210/jcem-60-1-174. [DOI] [PubMed] [Google Scholar]
- 29.Hemsell DL, Grodin JM, Brenner PF, Siiteri PK, MacDonald PC. Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. J Clin Endocrinol Metab. 1974;38:476–479. doi: 10.1210/jcem-38-3-476. [DOI] [PubMed] [Google Scholar]
- 30.Burrows H. The occurrence of scrotal hernia in mice under treatment with oestrin. Br J Surg. 1934;21:507–512. [Google Scholar]
- 31.Rohrmann S, et al. The prevalence of low sex steroid hormone concentrations in men in the Third National Health and Nutrition Examination Survey (NHANES III) Clin Endocrinol (Oxf) 2011;75:232–239. doi: 10.1111/j.1365-2265.2011.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shores MM, Matsumoto AM. Testosterone, aging and survival: Biomarker or deficiency. Curr Opin Endocrinol Diabetes Obes. 2014;21:209–216. doi: 10.1097/MED.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, et al. A humanized pattern of aromatase expression is associated with mammary hyperplasia in mice. Endocrinology. 2012;153:2701–2713. doi: 10.1210/en.2011-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012;77:27–35. doi: 10.1016/j.steroids.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada N, Utsumi T, Takagi Y. Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc Natl Acad Sci USA. 1993;90:11312–11316. doi: 10.1073/pnas.90.23.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brodie A, Inkster S, Yue W. Aromatase expression in the human male. Mol Cell Endocrinol. 2001;178:23–28. doi: 10.1016/s0303-7207(01)00444-0. [DOI] [PubMed] [Google Scholar]
- 38.Simpson ER. Sex, fat and breast cancer. Gynecol Endocrinol. 2009;25:1. doi: 10.1080/09513590802296153. [DOI] [PubMed] [Google Scholar]
- 39.Krege JH, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lubahn DB, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olde B, Leeb-Lundberg LM. GPR30/GPER1: Searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20:409–416. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Feder D, et al. Hormonal receptors in skeletal muscles of dystrophic mdx mice. BioMed Res Int. 2013;2013:604635. doi: 10.1155/2013/604635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunbier AK, et al. Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor-positive breast cancer in postmenopausal women. J Clin Oncol. 2010;28:1161–1167. doi: 10.1200/JCO.2009.23.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Z, et al. Adenosine A1 receptor, a target and regulator of estrogen receptoralpha action, mediates the proliferative effects of estradiol in breast cancer. Oncogene. 2010;29:1114–1122. doi: 10.1038/onc.2009.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moy I, et al. Estrogen-dependent sushi domain containing 3 regulates cytoskeleton organization and migration in breast cancer cells. Oncogene. 2015;34:323–333. doi: 10.1038/onc.2013.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro-Rivera E, Samudio I, Safe S. Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J Biol Chem. 2001;276:30853–30861. doi: 10.1074/jbc.M103339200. [DOI] [PubMed] [Google Scholar]
- 47.Iwano M, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shozu M, et al. Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. N Engl J Med. 2003;348:1855–1865. doi: 10.1056/NEJMoa021559. [DOI] [PubMed] [Google Scholar]
- 49.Lu C, Luo J. Decoding the androgen receptor splice variants. Transl Androl Urol. 2013;2:178–186. doi: 10.3978/j.issn.2223-4683.2013.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahrens-Fath I, Politz O, Geserick C, Haendler B. Androgen receptor function is modulated by the tissue-specific AR45 variant. FEBS J. 2005;272:74–84. doi: 10.1111/j.1742-4658.2004.04395.x. [DOI] [PubMed] [Google Scholar]
- 51.Weiss B, Faus H, Haendler B. Phylogenetic conservation of the androgen receptor AR45 variant form in placental mammals. Gene. 2007;399:105–111. doi: 10.1016/j.gene.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 52.Hu DG, et al. Identification of androgen receptor splice variant transcripts in breast cancer cell lines and human tissues. Horm Cancer. 2014;5:61–71. doi: 10.1007/s12672-014-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitani Y, et al. Alterations associated with androgen receptor gene activation in salivary duct carcinoma of both sexes: Potential therapeutic ramifications. Clin Cancer Res. 2014;20:6570–6581. doi: 10.1158/1078-0432.CCR-14-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, et al. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142:2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- 55.Weihua Z, et al. Update on estrogen signaling. FEBS Lett. 2003;546:17–24. doi: 10.1016/s0014-5793(03)00436-8. [DOI] [PubMed] [Google Scholar]
- 56.Younes M, Honma N. Estrogen receptor β. Arch Pathol Lab Med. 2011;135:63–66. doi: 10.5858/2010-0448-RAR.1. [DOI] [PubMed] [Google Scholar]
- 57.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: Its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu I, Vitkus S, Da J, Yeh S. Role of oestrogen receptors in bladder cancer development. Nat Rev Urol. 2013;10:317–326. doi: 10.1038/nrurol.2013.53. [DOI] [PubMed] [Google Scholar]
- 59.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonéy-Montoya J, Ziegler YS, Curtis CD, Montoya JA, Nardulli AM. Long-range transcriptional control of progesterone receptor gene expression. Mol Endocrinol. 2010;24:346–358. doi: 10.1210/me.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohammed H, et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 2013;3:342–349. doi: 10.1016/j.celrep.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agarwal VR, et al. Molecular basis of severe gynecomastia associated with aromatase expression in a fibrolamellar hepatocellular carcinoma. J Clin Endocrinol Metab. 1998;83:1797–1800. doi: 10.1210/jcem.83.5.4773. [DOI] [PubMed] [Google Scholar]
- 63.Aida-Yasuoka K, et al. Estradiol promotes the development of a fibrotic phenotype and is increased in the serum of patients with systemic sclerosis. Arthritis Res Ther. 2013;15:R10. doi: 10.1186/ar4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo N, et al. Estrogen-mediated activation of fibroblasts and its effects on the fibroid cell proliferation. Transl Res. 2014;163:232–241. doi: 10.1016/j.trsl.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 65.Ng CW, et al. Extensive changes in DNA methylation are associated with expression of mutant huntingtin. Proc Natl Acad Sci USA. 2013;110:2354–2359. doi: 10.1073/pnas.1221292110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gopaul R, Knaggs HE, Lephart ED. Biochemical investigation and gene analysis of equol: A plant and soy-derived isoflavonoid with antiaging and antioxidant properties with potential human skin applications. Biofactors. 2012;38:44–52. doi: 10.1002/biof.191. [DOI] [PubMed] [Google Scholar]
- 67.Shah YM, Basrur V, Rowan BG. Selective estrogen receptor modulator regulated proteins in endometrial cancer cells. Mol Cell Endocrinol. 2004;219:127–139. doi: 10.1016/j.mce.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Stephens SB, Chahal N, Munaganuru N, Parra RA, Kauffman AS. Estrogen stimulation of Kiss1 expression in the medial amygdala involves estrogen receptor-α but not estrogen receptor-β. Endocrinology. 2016;157:4021–4031. doi: 10.1210/en.2016-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan O, Ornek T, Seval Y, Sati L, Arici A. Tenascin is highly expressed in endometriosis and its expression is upregulated by estrogen. Fertil Steril. 2008;89:1082–1089. doi: 10.1016/j.fertnstert.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 70.Tan S, et al. Identification of miR-26 as a key mediator of estrogen stimulated cell proliferation by targeting CHD1, GREB1 and KPNA2. Breast Cancer Res. 2014;16:R40. doi: 10.1186/bcr3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker G, et al. Estrogen-regulated gene expression predicts response to endocrine therapy in patients with ovarian cancer. Gynecol Oncol. 2007;106:461–468. doi: 10.1016/j.ygyno.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Chung D, Gao F, Jegga AG, Das SK. Estrogen mediated epithelial proliferation in the uterus is directed by stromal Fgf10 and Bmp8a. Mol Cell Endocrinol. 2015;400:48–60. doi: 10.1016/j.mce.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prest SJ, May FE, Westley BR. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J. 2002;16:592–594. doi: 10.1096/fj.01-0498fje. [DOI] [PubMed] [Google Scholar]
- 74.Mahendroo MS, Cala KM, Hess DL, Russell DW. Unexpected virilization in male mice lacking steroid 5 alpha-reductase enzymes. Endocrinology. 2001;142:4652–4662. doi: 10.1210/endo.142.11.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Rooy C, Grossmann M, Zajac JD, Cheung AS. Targeting muscle signaling pathways to minimize adverse effects of androgen deprivation. Endocr Relat Cancer. 2016;23:R15–R26. doi: 10.1530/ERC-15-0232. [DOI] [PubMed] [Google Scholar]
- 76.Pihlajamaa P, Sahu B, Jänne OA. Determinants of receptor- and tissue-specific actions in androgen signaling. Endocr Rev. 2015;36:357–384. doi: 10.1210/er.2015-1034. [DOI] [PubMed] [Google Scholar]
- 77.Long BJ, et al. Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. J Natl Cancer Inst. 2004;96:456–465. doi: 10.1093/jnci/djh076. [DOI] [PubMed] [Google Scholar]
- 78.Chen D, et al. Prostaglandin E(2) induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH(2)-terminal kinase in adipose fibroblasts. Cancer Res. 2007;67:8914–8922. doi: 10.1158/0008-5472.CAN-06-4751. [DOI] [PubMed] [Google Scholar]
- 79.Zhao H, et al. A novel promoter controls Cyp19a1 gene expression in mouse adipose tissue. Reprod Biol Endocrinol. 2009;7:37. doi: 10.1186/1477-7827-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA microarray data that support the findings of this study have been deposited to the National Center for Biotechnology Information Gene Expression Omnibus database under the accession no. GSE92748 (https://www.ncbi.nlm.nih.gov/geo/).