Fig. 1.
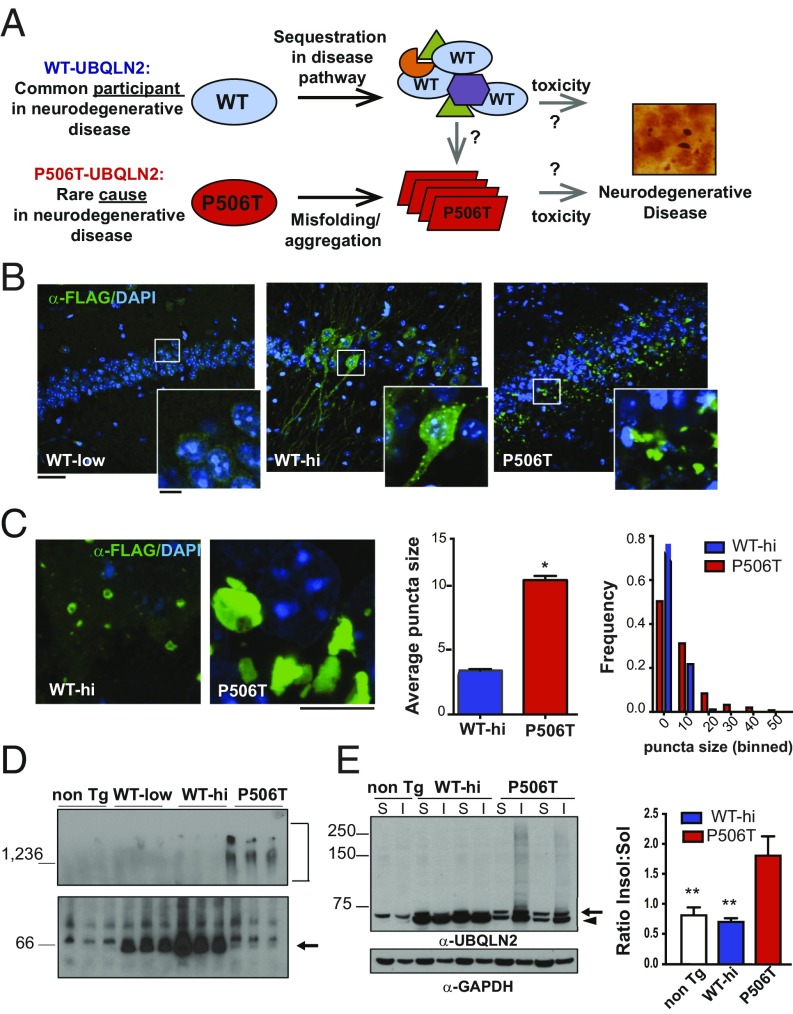
Transgenic mice show that the intrinsic propensity of UBQLN2 to aggregate is enhanced by a pathogenic mutation (P506T). (A) Reminiscent of other disease-linked proteins, UBQLN2 commonly accumulates in neurodegenerative diseases and, when mutated, directly causes neurodegeneration along the ALS/FTD spectrum. Studies reported here seek to explain this behavior. (B) Immunofluorescence of the hippocampus in UBQLN2 transgenic mouse lines expressing FLAG-tagged WT-UBQLN2 or P506T-UBQLN2 under control of the mouse prion promoter (3-mo old). Tissue sections were stained for FLAG-UBQLN2 (green) and DAPI (blue). Higher-magnification Insets highlight differences in puncta/aggregate formation by WT- and P506T-UBQLN2. (Scale bars, 50 μM; 10 μM Insets.) (C) Neuronal puncta/aggregates in WT-hi and P506T mouse lines differ in size and appearance. (Scale bars, 5 μM.) (Right) Quantification of puncta/aggregate size in WT-UBQLN2-hi and P506T-UBQLN2 hippocampal neurons, plotted as mean size compared by unpaired t test, (error bars = SE, *P < 0.05), and as relative distribution of puncta size (n = 5 WT-hi mice, 776 total puncta: n = 6 P506T mice, 3,745 total puncta). The WT and P506T puncta size distributions differ significantly based on the Kolmogorov–Smirnov test (D = 0.26402, P < 2.2e-16). (D) Native-PAGE of brain lysates from nontransgenic or transgenic mice probed with anti-UBQLN2 antibody. Bracket indicates high molecular-weight species present only in P506T-UBQLN2 mice, and arrow indicates UBQLN2 monomer. (E) Western blot of soluble (S) and insoluble (I) brain fractions from transgenic and nontransgenic mice probed with anti-UBQLN2 antibody (arrowhead, FLAG-UBLQN2; arrow, murine UBLQN2). (Right) Average insoluble/soluble ratio for UBQLN2 monomer in each line (**P < 0.005).