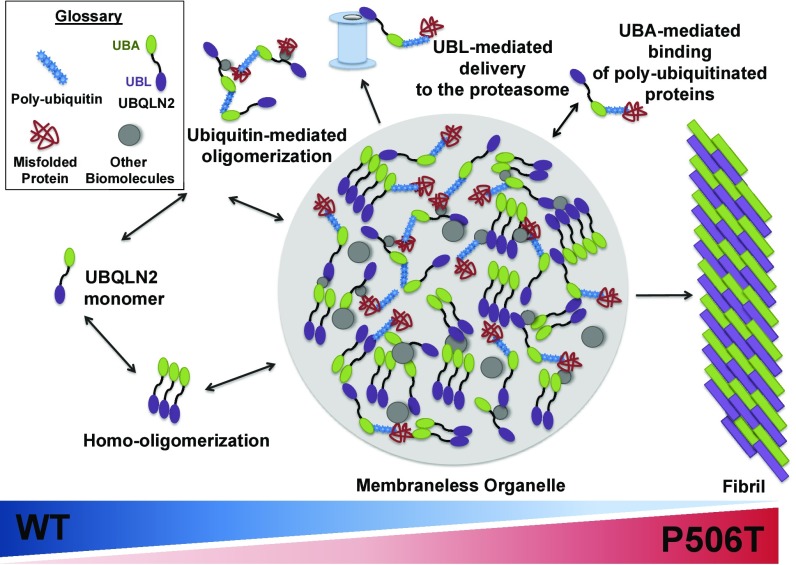
Fig. 8.
Schematic of dynamic continuum between oligomerization, liquid–liquid phase transition and fibril formation for WT and mutant UBQLN2. In this speculative model, UBQLN2 forms oligomers with itself and associates with ubiquitinated substrates and other biomolecules, including chaperones. While the UBA and UBL domains of UBQLN2 may interact intermolecularly, this model is not meant to imply particular structures for oligomeric states of UBQLN2. Under physiological conditions, UBQLN2 oligomers assemble into membraneless organelles that function as reservoirs for UPS components, thereby facilitating chaperone-mediated folding or proteasomal degradation of UBQLN2-bound substrates. The gradients (Bottom) reflect the potential shift in equilibrium from dynamic molecular assemblies (Left) to more stable hydrogels or amyloid fibrils (Right) in the presence of disease-causing mutations (such as P506T) or proteotoxic stress.