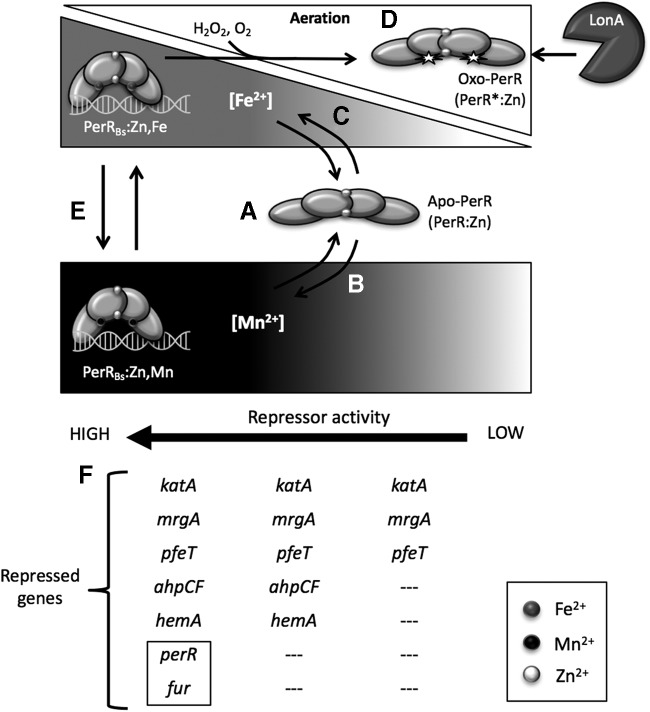
FIG. 3.
Mechanism of gene regulation by PerRBs. (A) Apo-PerR (PerR:Zn) lacks a metal cofactor at its metal-sensing site. This causes PerR to adopt a conformation that prevents it from binding to its DNA operator sites. As a result, no gene repression takes place. (B) As Mn2+ concentrations increase, apo-PerR becomes metallated, triggering a conformational change in PerR:Zn,Mn allowing it to bind its specific operator sites and repress PerR-regulated genes. Note that PerR:Zn,Mn is insensitive to oxidizing agents. (C) As Fe2+ concentrations increase, apo-PerR becomes metallated by iron. PerR:Zn,Fe binds to PerR operator sites and repress genes in the PerR regulon. However, during aerobic growth or in the presence of ROS, the ability of PerR:Zn,Fe to act as a repressor is limited by protein oxidation. (D) Oxidation of PerR exposes a conserved signature residue sequence recognized by LonA. Thus, oxo-PerR is targeted for degradation by the protease, preventing accumulation of inactive protein. (E) Metallated PerR can reversibly bind to Mn2+ or Fe2+ as metal concentrations in the cell vary. (F) Overview of the ability of PerR to repress various genes as a function of metal availability. In the presence of iron, under aerobic conditions, inactivation of PerR prevents accumulation of high levels of active repressor. As a result, metallated PerR:Zn,Fe is most efficient at repression of genes postulated to have high-affinity operator sites (katA, mrgA, pfeT). PerR:Zn,Mn, which is not susceptible to oxidation, can accumulate to a high effective level and strongly represses genes the entire PerR regulon, including those genes postulated to have the lowest affinity operator sites (perR, fur) (as highlighted by box). ROS, reactive oxygen species.