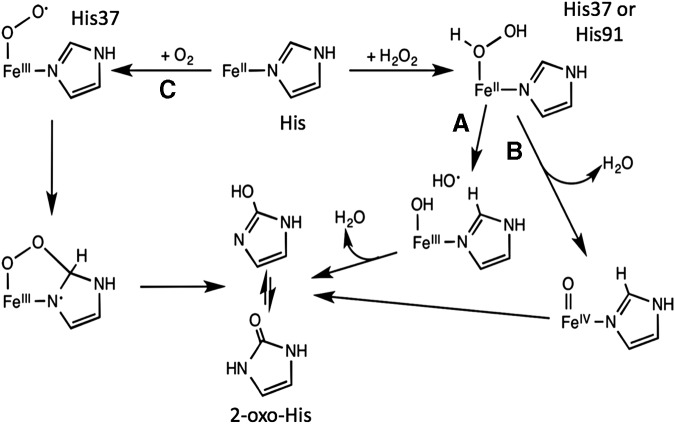
FIG. 4.
Metal-catalyzed oxidation of PerR. Formation of 2-oxo-histidine of PerRBs:Zn,Fe site 2 via three putative pathways. His37 or His91 can both become oxidized when exposed to H2O2. This can lead to either (A) the generation of a hydroxyl radical via oxidation of Fe2+ into Fe3+ as a result of the homolytic cleavage of the O-O of the peroxido intermediate or (B) the production of a high valent Fe4+ ion (especially under pH ∼7.0) caused by the heterolytic cleavage of that same bond. Conversely, (C) in the presence of O2, His37 is specifically targeted, resulting in a superoxo-Fe3+ intermediate. Subsequent bond formation and O-O bond cleavage of the end derivative leads to the final production of 2-oxo-His [figure adapted from (100)].