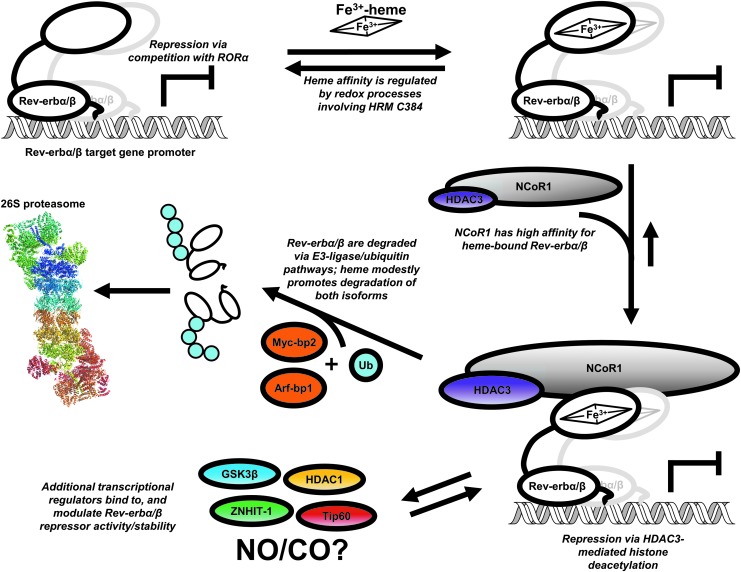
FIG. 4.
Heme-dependent repression and degradation of Rev-erbα/β. One mode of Rev-erbα/β repression is through competition with RORα for binding to its response element (ROR-RE) within the promoters of target genes. Rev-erbs can bind as monomers and homodimers to Rev-REs, although recent studies suggest that they may also form heterodimers (8, 13, 31). Although Fe3+-heme binds to Rev-erbβ with high affinity (Kd ≤0.1 nM), redox processes that cause dissociation of the Fe3+-heme axial thiolate ligand (including thiol-disulfide interconversion and Fe3+- to Fe2+-heme reduction) cause Kd to increase to ≥14 nM (11). Since the concentration of RH in the nucleus is ≤2.5 nM (30), loss of the HRM heme axial thiolate would cause dissociation of heme. Coimmunoprecipitation studies show that the NCoR1-HDAC3 complex has a high affinity for heme-bound Rev-erbα/β (70, 102); once bound, the complex leads to target gene repression via histone deacetylation. Additional regulators of Rev-erbβ repressor activity have been identified, including Tip60, an acetyl transferase, HDAC1, a histone deacetylase, and ZNHIT-1, a zinc-finger containing protein; GSK3β has also been shown to regulate the stability of the α-isoform (see main text for references); whether heme plays a role in regulating interactions of these proteins with Rev-erbs is unknown. Gaseous signaling molecules, NO and CO may also regulate the repressor function of Rev-erbs by binding to heme; however, additional studies are required to support this hypothesis. Although heme facilitates interaction of NCoR1 with Rev-erbs, it also appears to promote proteasomal degradation of both isoforms through a ubiquitin-dependent pathway involving E3-ligases Arf-bp1 and Myc-bp2. CO, carbon monoxide; HDAC1, histone deacetylase 1; NO, nitric oxide; RH, regulatory heme; RORα, RAR-related orphan receptor α.