FIG. 5.
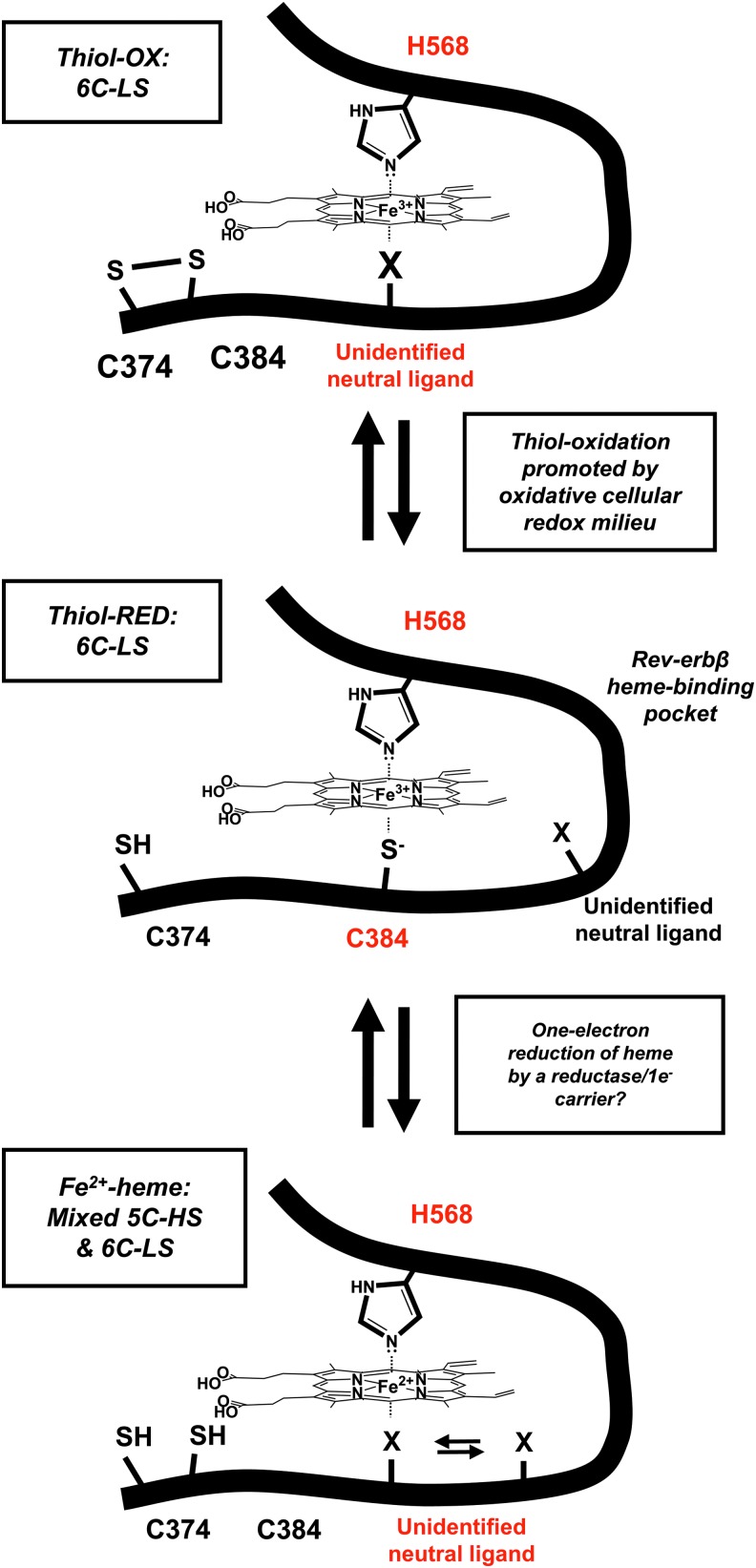
Rev-erbβ undergoes redox-mediated heme ligand switching. The thiol-reduced form of the Rev-erbβ LBD (thiol-RED; C374 and C384 exist as thiols/thiolates) binds Fe3+-heme in a 6C-LS complex with H568 and C384 axial ligands and a Kd of ∼100 pM. Under oxidizing conditions, C384 forms a disulfide with neighboring C374 (thiol-OX), leading to a 6C-LS system where an unidentified neutral ligand assumes the position once occupied by the thiolate. Thiol-OX has a Kd for Fe3+-heme of ≥14 nM, well above the nuclear RH level of ≤2.5 nM, suggesting that thiol-OX would exist as an apoprotein in the nucleus. Our working hypothesis is that C374–C384 thiol-disulfide interconversion is in equilibrium with the GSH:GSSG or cysteine:cystine couples and reflects the redox poise of the nucleus. Similarly, the one-electron reduction of Fe3+- to Fe2+-heme is accompanied by dissociation of the HRM heme axial thiolate, leading to a mixed 5C-HS/6C-LS system where an unidentified neutral ligand (presumably the same ligand involved in the thiol-RED/OX ligand switch) is loosely associated with heme. Although it is unclear what heme redox state is relevant to Rev-erbβ in the cell, it is intriguing to consider one-electron reductants that could interface with Rev-erbβ, such as ferredoxins or cytochrome P450 reductase, although nuclear localization of the reductant is presumably required. 5C-HS, five-coordinate high spin; 6C-LS, six-coordinate low-spin.