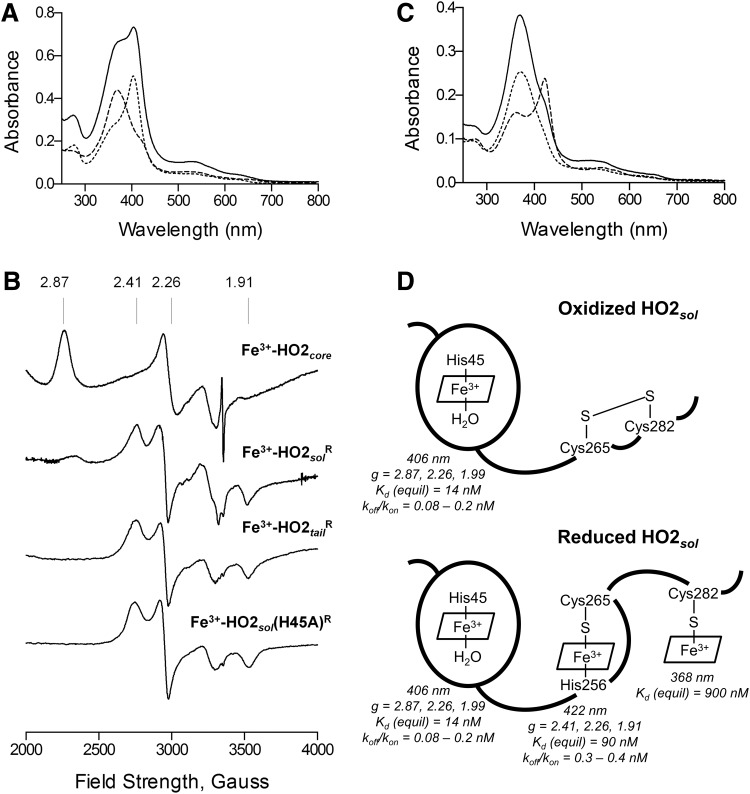
FIG. 6.
Spectral characterization of HO2 in the Fe3+-heme-bound forms. (A) Absorbance spectra of 5 μM Fe3+-heme-bound forms of HO2core (…), HO2tailR ( ), and HO2solR (—) in 50 mM Tris (pH 8.0) and 50 mM KCl at 20°C. (B) EPR spectra of the Fe3+-heme-bound forms of HO2core, HO2solR, HO2tailR, and the His45Ala variant of HO2solR. The g values are indicated above the spectra. (C) Absorbance spectra of 5 μM Fe3+-heme-bound forms of HO2tailR (—), HO2tail(C282A)R (
), and HO2solR (—) in 50 mM Tris (pH 8.0) and 50 mM KCl at 20°C. (B) EPR spectra of the Fe3+-heme-bound forms of HO2core, HO2solR, HO2tailR, and the His45Ala variant of HO2solR. The g values are indicated above the spectra. (C) Absorbance spectra of 5 μM Fe3+-heme-bound forms of HO2tailR (—), HO2tail(C282A)R ( ), and HO2tail(C265A)R (…) in 50 mM Tris (pH 8.0) and 50 mM KCl at 20°C. (D) A model of heme binding to the oxidized form of HO2sol, which has a single heme-binding site due to the participation of Cys265 and Cys282 in a disulfide bond, and the reduced form of HO2sol, which has three heme-binding sites. Below each heme-binding site are the characteristics of that site, including the maximum absorbance, the g-values, the Kd values obtained by equilibrium titrations [Kd (equil)], and the koff/kon values obtained by kinetic measurements. The figures in (A–C) are reprinted (adapted) with permission from Fleischhacker et al. (20). Copyright (2015) American Chemical Society. EPR, electron paramagnetic resonance; HO2core, HO2 spanning residues 1-248; HO2R, HO2 in the disulfide bond-reduced form; HO2sol, HO2 spanning residues 1-288; HO2tail, HO2 spanning residues 213-288. EPR, electron paramagnetic resonance; HO2core, HO2 spanning residues 1-248; HO2R, HO2 in the disulfide bond-reduced form; HO2sol, HO2 spanning residues 1-288; HO2tail, HO2 spanning residues 213-288.
), and HO2tail(C265A)R (…) in 50 mM Tris (pH 8.0) and 50 mM KCl at 20°C. (D) A model of heme binding to the oxidized form of HO2sol, which has a single heme-binding site due to the participation of Cys265 and Cys282 in a disulfide bond, and the reduced form of HO2sol, which has three heme-binding sites. Below each heme-binding site are the characteristics of that site, including the maximum absorbance, the g-values, the Kd values obtained by equilibrium titrations [Kd (equil)], and the koff/kon values obtained by kinetic measurements. The figures in (A–C) are reprinted (adapted) with permission from Fleischhacker et al. (20). Copyright (2015) American Chemical Society. EPR, electron paramagnetic resonance; HO2core, HO2 spanning residues 1-248; HO2R, HO2 in the disulfide bond-reduced form; HO2sol, HO2 spanning residues 1-288; HO2tail, HO2 spanning residues 213-288. EPR, electron paramagnetic resonance; HO2core, HO2 spanning residues 1-248; HO2R, HO2 in the disulfide bond-reduced form; HO2sol, HO2 spanning residues 1-288; HO2tail, HO2 spanning residues 213-288.