Abstract
Malignant astrocytomas are aggressive cancers of glial origin that can develop into invasive brain tumors. The disease has poor prognosis and high recurrence rate. Astrocytoma cell lines of human origin are an important tool in the experimental pathway from bench to bedside because they afford a convenient intermediate system for in vitro analysis of brain cancer pathogenesis and treatment options. We undertook the current study to determine whether hydrogel culture methods could be adapted to support the growth of astrocytoma cell lines, thereby facilitating a system that may be biologically more similar to in vivo tumor tissue. Our experimental protocols enabled maintenance of Grade IV astrocytoma cell lines in conventional monolayer culture and in the extracellular matrix hydrogel, Geltrex™. Light and fluorescence microscopy showed that hydrogel environments promoted cellular reorganization from dispersed cells into multilayered aggregates. Transmission electron microscopy revealed the prevalence of autophagy and nuclear membrane distortions in both culture systems. Analysis of microarray Gene Expression Omnibus (GEO) DataSets highlighted expression of genes implicated in pathways for cancer progression and autophagy. A pilot quantitative polymerase chain reaction (qPCR) analysis of the autophagic biomarkers, Beclin 1 (BECN1) and microtubule-associated proteins 1A/1B light chain 3B (MAP1LC3B), with two reference genes (beta actin, ACTB; glyceraldehyde 3-phosphate dehydrogenase, GAPDH), uncovered a relative increase of BECN1 and LC3B in hydrogel cultures of astrocytoma as compared to the monolayer. Taken together, results establish that ultrastructural and molecular characteristics of autophagy are features of this astrocytoma cell line, and that hydrogel culture systems can afford novel opportunities for in vitro studies of glioma.
Keywords: astrocytoma, autophagy, BECN1, cancer, glia, glioblastoma, hydrogel, LC3B, qPCR, transmission electron microscopy
1. INTRODUCTION
Glial brain tumors account for 42% of all central nervous system neoplasms, with 75% of these classified as malignant [1]. Astrocytomas are tumors of glial origin that can impair brain function and compromise mental health, and globally affect more men than women [2]. Malignant astrocytomas are aggressive cancers with poor clinical outcomes that beckon for greater treatment options [3]. Even with decades of research and treatment trials, the current standards in treatment are aimed at providing palliative care. Malignant gliomas have an ability to acquire resistance to therapeutic modalities and are inherently aggressive due to their aberrant proliferation, reduced apoptosis, avoidance of external and growth control and immunoregulation [4].
As molecular biomarkers and cell signaling pathways are identified in gliomas, targeted therapies are becoming more promising and many platforms for developing and testing interventions use in vitro models [5]. Cell culture systems offer a cost-effective approach to study complex cellular processes such as migration, differentiation, angiogenesis and tissue folding [6]. Cell culture systems are an essential and easily manipulated tool in the cancer research experimental repertoire. Traditionally, cell culture has been conducted on flat and rigid substrates, which are engineered to allow cell adhesion and propagation in what is known as a two-dimensional (2D) monolayer system. However, cells in tissues and organs grow next to, and on top of one other, in a multilayered three-dimensional (3D) fashion and are surrounded by a complex extracellular matrix as well as diffusible factors [7]. The cell’s microenvironment plays a critical role in determining cellular morphology and gene expression levels, which in turn can drive cancer progression [8, 9]. There is increasing awareness that 2D monolayer culture systems may not adequately provide predictive data with respect to the response of cells to anti-cancer drugs because they lack the complex tissue organization of the in vivo systems [9, 10].
Tissue engineering is a powerful methodology that enables the design of experimental systems to identify treatments for disorders such as cancer, neurodegenerative diseases and cardiovascular diseases [11]. Novel cell culture platforms have been developed using tissue engineering approaches through directed integration of living cells, scaffold materials and essential growth factors [12]. Hydrogel scaffolds are becoming increasingly popular for tissue culture due to their biocompatibility and resemblance to the naturally occurring extracellular matrices, which allow for optimal cell growth, migration and survival [13, 14]. For these reasons, tissue engineering with matrix hydrogels provides a promising alternative for traditional in-vitro 2D models. Currently, culture platforms that incorporate hydrogels are being used in cancer research for screening anti-cancer compounds [15–17]. Reports suggest that these hydrogel systems more closely resemble cell-cell and cell-matrix interactions occurring in native tumor tissue, for example, through promotion of cell aggregation and spheroid formation [13], [18–20].
In the current study, we evaluated a hydrogel culture system for its ability to facilitate the growth and survival of CCF-STTG1, a cell line that was derived from a (female) donor diagnosed with Grade IV astrocytoma [21]. A subset of experiments were designed to permit comparisons with LN-18, a (male) Grade IV astrocytoma cell line that has been used in prior studies to determine the impact of the tissue culture environment on cell morphology, and for structural investigations of autophagy [23]. Experiments presented here examined the growth of CCF-STTG1 and LN-18 under conventional cell culture conditions (monolayer) and with hydrogel. The matrix environment was provided using Geltrex™, a hydrogel prepared from murine Engelbreth-Holm-Swarm tumors and containing a proprietary mix of structural proteins and growth factors vital for cell growth and differentiation [24]. The structural organization of CCF-STTG1 and LN-18 cells in monolayer and hydrogel environments was visualized using light and fluorescence microscopy. Additional experiments were undertaken to visualize the morphology of the CCF-STTG1 cells using transmission electron microscopy (TEM) and outcomes were compared with those from previous investigations with LN-18 [23]. We used a data mining approach for in silico identification of the autophagy genes from the Human Autophagy Database (HADb) in Gene Expression Omnibus (GEO) microarray data for the CCF-STTG1 cell line and compared outcomes to those from a GEO DataSet for a normal human astrocyte cell line. Autophagy genes retrieved from the microarray data were further examined through ontological and pathway analysis. Quantitative PCR (qPCR) analysis of Beclin 1 (BECN1) and microtubule-associated proteins 1A/1B light chain 3B (MAP1LC3B), two key autophagy biomarker genes, was completed in the two culture conditions.
Our results showed that the Grade IV astrocytoma cell lines, CCF-STTG1 and LN-18, can be maintained in conventional monolayer culture on a rigid substrate, and in the extracellular matrix hydrogel, Geltrex™. We observed that cellular reorganization from dispersed cells to cell aggregates is enabled in the hydrogel for both cell lines. Structural features such as autophagy and nuclear envelope distortions are present in both conditions for CCF-STTG1, consistent with prior observations in LN-18 cells [23]. TEM imaging indicates that CCF-STTG1 cells migrate into the hydrogel from the surface onto which they are seeded. Analysis of 222 HADb genes implicated in autophagy pathways in open source datasets for CCF-STTG1 and normal astrocytes suggests a subset of autophagy genes are expressed in both cell lines when cultured under monolayer conditions. qPCR experiments showed increase in the relative expression of the autophagy biomarkers, BECN1 and LC3B, in hydrogel cultures of CCF-STTG1 as compared to monolayer culture in experiments with the two reference genes, beta actin (ACTB) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Taken together, these findings may be useful for the future design and identification of intervention strategies against astrocytoma.
2. METHODS
2.1 Cell Culture
The CCF-STTG1 cells (ATCC® CRL-1718™; Lot #s 58033362 and 61318750) were cultured according to the vendor’s instructions (ATCC; Manassas, VA) using RPMI-1640 medium (ATCC, Lot # 62285353) supplemented with 10% fetal bovine serum (FBS; Gibco, Lot #1025354). The LN-18 cell line (ATCC® CRL-2610™; Lot #61978349) was cultured as per the vendor’s instructions (ATCC) using DMEM medium (ATCC, Lot #64017510) supplemented with 5% FBS. The culture media were replenished every 48 h and the cell growth was monitored at regular intervals using an inverted Nikon Diaphot TMD phase-contrast microscope. At ~80% confluence, the cells were dislodged with 0.25% trypsin-EDTA solution (Sigma-Aldrich; T4049). Three stock ampules (passage 1) were frozen for each lot of cells until experimentation.
The CCF-STTG1 cells for live cell imaging, epifluorescence microscopy and TEM were prepared by thawing a previously frozen stock ampule (Lot # 58033362, Passage 1) in a T25 flask. The cells were trypsinized at 80% confluency and titered using a hemocytometer. The cells were plated at passage 2 at a seeding density of 5 × 104 cells per cm2 in 2 slides, 4 chambers each. One slide was used for fluorescence microscopy (VWR, Cat #62407-294) and the other for TEM (Permanox™; Nalge Nunc International, Rochester, NY, Cat #62407-330). The LN-18 cells were prepared for live cell imaging and epifluorescence microscopy using the cell culture and sample preparation protocol described above. Two chambers on each slide were used for conventional monolayer culture. The other two chambers were used for 3D hydrogel culture in LDEV-Free Reduced Growth Factor Basement Membrane Matrix - Geltrex™ (Invitrogen; Cat # A1413202). The experiment was repeated three times using the cell culture and sample preparation protocol above. CCF-STTG1 cells intended for live cell imaging and RNA isolation (Lot # 61318750, Passage 1) were thawed in a T25 flask and passaged as described above. The cells were plated at passage 2 in 2 T25 flasks – 1 for monolayer and other for hydrogel culture. The experiment was repeated three times using the above protocol.
2.2 Matrix Preparation
Geltrex™ was stored at −80°C and thawed at 4°C one day before its intended use according to vendor instructions The hydrogel solution was mixed well by trituration with a pipette, then added to the slide chambers and T25 flasks (100 μl per cm2) under sterile conditions. The slides and T25 flasks were incubated at 37°C for 30 minutes to allow the solidification of matrix. Cells were added to the hydrogel at a density of 5 x 104 cells/cm2 and allowed to grow for 48 hours at 37°C and 5% CO2 to ~80% confluence before capturing images using epifluorescence and transmission electron microscopy.
2.3 Fluorescent Probes
Monolayer and hydrogel cultures were prepared for epifluorescence microscopy. One chamber from each condition was labeled with fluorescent probes and the other was fixed but not labeled to serve as an auto-fluorescence control. Fixation and fluorescent labeling were undertaken at room temperature (~ 70°F) unless otherwise indicated. The cells were washed once for 2 minutes with 800 μl of serum-free media (RPMI1640; ATCC, Lot #62285353) followed by fixation using 4% paraformaldehyde (PFA; Electron Microscopy Sciences, CAS #30525-89-4) for 10 minutes. The cells were then rinsed twice for 2 minutes with 800 μl of 1X Phosphate buffered saline, pH 7.4 (PBS; Sigma-Aldrich, P3813), permeabilized for 20 minutes with 500 μl of 0.2% Triton X-100 (Sigma-Aldrich; Cat # T8787) in 1X PBS, then washed twice for 2 minutes with 800 μl of 1X PBS.
For F-actin visualization, cells in each chamber were incubated for 20 minutes in the dark in Alexa Fluor® 488 phalloidin dye (Invitrogen; Cat # A12379) diluted with 1% bovine serum albumin (BSA; Sigma-Aldrich, Cat # A2153) in 1X PBS. 5μl of 6.6 μM methanolic stock of Alexa Fluor® 488 phalloidin dye was added for every 200 μl of 1% BSA in 1X PBS. The auto-fluorescence control samples were treated with equal amounts of 1% BSA in 1X PBS during this procedure. The labelled cells were rinsed twice with 800 μl of 1X PBS, then incubated in the dark for 10 minutes with 500 μl of 1 μg/ml Hoechst 33342 (Invitrogen; Cat # H3570) solution in 1X PBS. The auto-fluorescence control samples were treated with equal amounts of 1X PBS during this procedure.
The chambers were rinsed twice with 800 μl of 1X PBS then processed with the SlowFade® AntiFade kit (Invitrogen, Cat # S2828). Briefly, cells were incubated in 300 μl of AntiFade Solution C for 15 minutes. The AntiFade solution C was removed and the sidewalls of the culture chambers were detached. This was followed by addition of a drop of AntiFade Solution A on the slide. The coverslip was gently placed on the slide to avoid the formation of air bubbles. The corners of the coverslip were sealed with nail polish to prevent the samples from drying.
2.4 Phase Contrast and Epifluorescence Imaging
Live monolayer and hydrogel cultures of CCF STTG1 cells were viewed using an inverted Nikon TE-2000 microscope integrated with a Coolsnap HQ CCD camera (Photometrics). Slides with fixed and labeled cells were viewed with the same platform using epifluorescence filter cube (B-2E/C or FITC) for Alexa Fluor® 488 phalloidin and UV-2A filter cube for Hoechst 33342.
TIFF images (1940 x 1460 pixels) were acquired using the Metavue image capture software (Molecular Devices) a 20X objective.
2.5 Quantification of Fluorescence Images
We assessed the characteristics of the cultures by counting the number of nuclei from fluorescent images of cells stained with Hoechst 33342 using the ‘multi-point tool’ in the ImageJ software platform [25]. The areas in the black background of composite images of cultures labelled with Hoechst 33342 and Alexa Fluor® 488 phalloidin, representing non-fluorescent regions without cells, were measured by selecting a thresholded region in the image using the Image J Measure tools. Data and statistical analysis are shown in Online Resource 2.
2.6 Transmission Electron Microscopy
Two resin blocks were prepared from each chamber comprising cells grown either in monolayer (6 chambers, 12 blocks) or hydrogel (6 chambers, 12 blocks). Fixation and sample processing was undertaken at room temperature (~ 70°F) unless otherwise indicated. Cells in Permanox™ slides were fixed for 16 hours in 1.5 ml of 2.5% glutaraldehyde (Electron microscopy sciences, CAS #111-30-8) / 0.1 M cacodylate buffer (Electron microscopy sciences, #11650) at 4°C, then rinsed twice (10 minutes each) with 1 ml of 0.1 M in cacodylate buffer. The cells were post-fixed for 2 hours by incubation in 1 ml of 2% osmium tetroxide (Electron microscopy sciences, CAS #20816-12-0) / 0.1M cacodylate buffer. The cells were washed with 1.5 ml of ddH2O for 15 minutes, then dehydrated consecutively in 50%, 70% and 95% ethyl alcohol (1 ml per chamber; 2X, 10 minutes each), followed by exposure to 100% ethyl alcohol (1 ml per chamber; 3X, 10 minutes each). Cells then were treated with 1 ml per chamber of the transitional solvent – a mixture of acetone : ethanol (2:3) 3 times (10 minutes each).
Fixed dehydrated samples were infiltrated with a mixture of absolute ethanol : EMbed 812 resin (Electron Microscopy Sciences, #14120) in 1:1 and 1:3 proportion (1 ml per chamber) for 30 minutes each, then exposed to pure resin for 15 minutes. The resin was replaced with fresh resin to fill the slide chambers to a height of ~2 mm. The slides were cured for 48 hours in a 60°C oven. After 48 hours, the chambers were removed from the slide and placed in liquid nitrogen to break away the Permanox™. The resin blocks were separated from the broken Permanox™ chambers with pliers. Resin blocks were cut into two pieces and each piece was double-embedded by arranging it cell-side down on a mold containing 100% EMbed 812 resin and curing for 48 hours in a 60°C oven. Grids were prepared from two resin blocks obtained from each chamber. A UC6 ultra-microtome (Leica Microsystems Inc., Buffalo Grove, IL) was used to prepare ultra thin sections of ~70 nm from resin blocks. Sections were collected on Formvar carbon grids (Electron microscopy sciences, Cat #FF100-Cu) and labelled using 2% aqueous Uranyl acetate solution in distilled water (Electron microscopy sciences, #22400).
A transmission electron microscope, H-7650 (Hitachi High-Technologies, Pleasanton, CA) equipped with an integrated CCD camera (AMT Corp., Woburn, MA) was used to view ultrastructural features of the cells. Accelerating voltages between 80kV and 120kV were used to examine grids and capture TIFF images (4000 x 3038 pixels).
2.7 Data Mining
Queries of the NCBI repository, GEO DataSet, retrieved one microarray dataset for the CCF-STTG1 cell line (GSM886923; 2012; Online Resource 3) and three datasets for a normal human astrocyte cell line (GSM397656-8; 2011; Online Resource 4), all arrayed on the Affymetrix Human Genome U133 Plus 2.0 Array chip that interrogates expression of over 47,000 transcripts and variants. The Affymetrix CEL files for GEO DataSet were downloaded from NCBI and submitted to the Array Analyis web-server [26] to retrieve ‘Present’, ‘Marginal’ and ‘Absent’ calls that, together with corresponding Ensembl IDs, were imported to Microsoft Excel. Gene expression data for PSIDs for HADb autophagy genes from the four datasets were analyzed for the common incidence of ‘Present’ (P) ‘Marginal’ (M) and ‘Absent’ (A) calls (Online Resource 5).
The PSIDs in the CCF-STTG1 microarray corresponding to HADb genes with a ‘Present’ call were submitted to the analysis wizard of The Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.8 [27]. ‘Gene List’ was selected as a list type and ‘Official Gene Symbol’ was selected as an identifier. The annotations were limited to the species ‘Homo sapiens’. Gene ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis for the autophagy-linked genes were completed using the functional annotation tool. Three GO terms (biological process, cellular component and molecular function) were used for analysis using medium stringency settings. GO results were summarized as the abundance of GO terms significantly associated with the gene list (P value < 0.05). KEGG pathways showing significant involvement of the autophagy-associated genes (P value < 0.05) were summarized in Online Resource 7. The KEGG pathways for ‘Regulation of autophagy’ and ‘Pathways in cancer (Glioma)’ were downloaded and shown as Fig. 5 and Fig. 6. Functional annotation clustering was undertaken for the genes with ‘Present’ calls and significant clusters (enrichment score > 1.3, P value < 0.05) were summarized in Online Resource 7.
Fig. 5. KEGG pathway analysis for CCF-STTG1.
HADb genes for PSIDs with a ‘Present’ call in GEO DataSet GSM886923 were involved in signaling pathways in the regulation of autophagy (P value < 0.05)
Fig. 6. KEGG pathway analysis for CCF-STTG1.
HADb genes for PSIDs with a ‘Present’ call in GEO DataSet GSM886923 were involved in glioma signaling pathways (P value < 0.05)
2.8 RNA Isolation and Quantitative PCR (qPCR)
RNA was isolated from monolayer and hydrogel cultures using TRIzol® Plus RNA Purification Kit according to vendor’s instructions (Ambion). DNase treatment of the RNA isolate to remove DNA contamination was achieved with the DNA-free™ kit from Ambion and according to manufacturer’s instructions. The quality of resulting RNA was evaluated using an Agilent 2100 bioanalyzer. Complementary DNA (cDNA) was prepared using 30 ng of RNA as a starting material and SuperScript® III First-Strand Synthesis System for RT-PCR (Invitrogen) according to vendor’s instructions. cDNA quality was analyzed using a Nanodrop Lite Spectrophotometer (Thermo Scientific) (Online Resource 8; Table 2) and polymerase chain reaction (PCR) using Platinum Hot Start PCR 2X Master Mix (Invitrogen) and primers for BECN1, LC3B, ACTB, and GAPDH genes (Integrated DNA Technologies) with sequences shown in Table 1. The PCR product sizes were analysed using 2% agarose gel electrophoresis (Online Resource 8, Fig. 3).
Table 2. Relative expression of BECN1 and LC3B in hydrogel as compared to monolayer cultures.
qPCR analysis of BECN1 and LC3B abundance in hydrogel and monolayer cultures was undertaken with reference genes ACTB and GAPDH. Shown in the table is the relative expression change of hydrogel (Treatment Group) vs monolayer (Calibrator) computed from 3 technical replicate wells for each experimental stock vial and represented as Mean ± Standard Error (S.E.). The guidelines recommended by Livak and Schmittgen were followed for calculating relative expression values [29]. (See Online Resource 8 for original data)
| Target/Reference Gene | BECN1/ACTB | LC3B/ACTB | BECN1/GAPDH | LC3B/GAPDH |
|---|---|---|---|---|
| Culture Vial 1 (n = 3) | 2.10 ± 0.07 | 4.72 ± 0.20 | 1.65 ± 0.05 | 1.66 ± 0.21 |
| Culture Vial 2 (n = 3) | 2.09 ± 0.2 | 3.39 ± 0.31 | 1.00 ± 0.16 | 2.03 ± 0.12 |
| Culture Vial 3 (n = 3) | 2.36 ± 0.26 | 3.46 ± 0.62 | 1.71 ± 0.17 | 1.46 ± 0.27 |
| Mean ± S.E. | 2.18 ± 0.09 | 3.86 ± 0.43 | 1.45 ± 0.23 | 1.72 ± 0.17 |
Table 1. Primers sequences for BECN1, LC3B, ACTB, and GAPDH genes.
cDNA quality was assessed with PCR using primers designed to overlap the locations of Taqman® assays for target (BECN1, LC3B) and reference (ACTB, and GAPDH) genes, which were used for qPCR analysis. PCR product size was analyzed using 2% agarose gel electrophoresis (Online Resource 8, Fig. 3)
| Gene Name | Primer Direction | 5’-3’ Primer Sequence | Primer Length (bp) | Amplicon Size (bp) |
|---|---|---|---|---|
| BECN1 | Forward | 5’ TGTTGCTGCTCCATGCTC 3’ | 18 | 107 |
| Reverse | 5’ AGATTTGTCTGTCAGAGACTCC 3’ | 22 | ||
| LC3B | Forward | 5’ CTGTTTCAGGTTTTGTCTGAG 3’ | 21 | 93 |
| Reverse | 5’ TTTCTCCCGCTGTACTCCA 3’ | 19 | ||
| ACTB | Forward | 5’ CGTCTTCCCCTCCATCGT 3’ | 18 | 63 |
| Reverse | 5’ TTCTGACCCATGCCCACC 3’ | 18 | ||
| GAPDH | Forward | 5’ TCTGCTCCTCCTGTTCGAC 3’ | 19 | 93 |
| Reverse | 5’ TCCGACCTTCACCTTCCCC 3’ | 19 |
Fig. 3. TEM of CCF-STTG1 cells maintained in monolayer and hydrogel cultures.
(Panels A-C) The nucleus (A,C,‘n’) and a few autophagic vacuoles (A–C, white arrowheads) could be seen in high resolution images of monolayer cultures. The cells adhered to each other and intercellular junctions could be identified between cells (B, white arrows). Nuclear envelope invaginations are shown in image A (black arrows) (Panels D–F) The cells cultured in hydrogel were closely associated with each other as shown in image F. Images show features of the nucleus (D,E,‘n’), intercellular junctions (F, white arrows) and autophagic vacuoles (D,E, white arrowheads). Nuclear envelope invaginations were observed as shown in image E and F (black arrows). Geltrex™ can be seen around the cells in the form of a grainy material (Fig. 3F). The images were captured from grids prepared from independent experiments conducted with cells cultured from different frozen stock sister ampules (A,D: Ampule 1; B,E: Ampule 2; C,F: Ampule 3). Scale bar = 800 nm (Figs. 3A–E), 2 μm (Fig. 3F)
qPCR was undertaken using Taqman® Universal Master mix II with UNG (Thermo Fisher, Cat #4440038), Taqman® Gene Expression Assays for BECN1 - Hs00186838_m1, GAPDH - Hs02758991_g1, MAP1LC3B – Hs00797944_s1 and ACTB - Hs01060665_g1, and 1:100 dilution of template cDNA. A total of four 96-well plates (BECN1-ACTB, BECN1-GAPDH, LC3B-ACTB and LC3B-GAPDH) were completed on StepOnePlus Real-Time PCR System (Applied Biosystems). A five-point 10-fold standard dilution series (1:5, 1:25, 1:125, 1:625, and 1:3125) was used to check the performance of each Taqman® assay using cDNA obtained from monolayer RNA sample. The relative standard curve method was used for relative quantification of BECN1 and LC3B gene expression. Parameters were set for precise quality control of qPCR assays. Plate data were analyzed only if three quality criteria were met: (1) slope between -3.3 to -3.6, (2) PCR efficiency between 80–110%, and (3) correlation coefficient (R2 value) > 0.99 for standard curves [28].
Each plate was processed with three technical replicates for each gene for each experimental stock vial of cells (n = 3 separate stock vials), for a total of 18 experimental samples (3 hydrogel treatment groups; 3 monolayer calibrator groups; 3 replicates each) along with a positive control, no template control (NTC) and no reverse transcriptase control (NRTC). BECN1 and LC3B gene expression was normalized to two reference genes, ACTB and GAPDH, with all 18 samples for each reference gene analysed on a separate plate (Online Resource 8). The data were analysed using CT values with the double delta CT method [29] (Online Resource 8).
2.9 Analysis of PUBMED publications using glioblastoma cell lines
The commercially available glioblastoma cell lines and gender of the respective donors were retrieved from the ATCC website [30]. PUBMED was queried with the commercial name of each cell line to identify research reports from the past 10 years. Data were analyzed for differences in the number of female versus male ATCC cell lines, as well as differences in the number of publication reports for female and for male cell lines (Online Resource 9).
2.10 Fig. Preparation
Phase contrast, epifluorescence and TEM pictures were assembled and labelled using Adobe Photoshop CS6. Minimal image processing was carried out to improve contrast using the ‘levels’ sliding bar. The image files were saved in TIFF format (3000 x 2258 pixels) at the resolution of 300 dpi. The graphs were prepared using Microsoft Excel 2010 and tables were prepared using Microsoft Word 2010.
2.11 Compliance with Ethical Standards
The CCF-STTG1 (ATCC® CRL-1718 ) and LN-18 (ATCC® CRL-2610 ) cell lines are offered by the vendor in compliance with national ethics standards. CCF-STTG1 and LN-18 cell lines are derived from female and male patients of Caucasian ethnicity, 68 and 65 years of age respectively [31, 32]. The cell lines are de-identified and can be validated with short tandem repeat profiling as confirmed by the vendor. The human origin of the CCF-STTG1 and LN-18 cell lines was verified by ATCC. The cell lines were cultured in the lab according to the vendor’s instructions and used within the first 3 passages and no longer than 3 months after receipt from the vendor. The use of the cell lines was approved by the NMSU Institutional Biosafety Committee (1401SE2F0103; “Gene Expression in the Nervous System”). Commercially available human cell lines from the ATCC repository are not considered human subjects research under the HHS human subjects regulations (45 CFR Part 46) and are not reviewed by the NMSU Institutional Review Board.
3. RESULTS
3.1 Cellular reorganization in hydrogel environments can be visualized with light microscopy
Phase contrast imaging of live cells allowed for visualization of distinguishing features of astrocytoma in monolayer and hydrogel systems (Fig. 1). In monolayer culture conditions, CCF-STTG1 (Fig. 1A,C,E) and LN-18 (Fig. 1G,I,K) cells assumed stellate or oblong-shapes, were dispersed on the slide substrate, and appeared phase-dark. In hydrogel, the CCF-STTG1 cells were closely associated with each other to form clusters (Fig. 1B,D,F). These clusters appeared to connect with the other clusters in the vicinity (Fig. 1F). LN-18 cells cultured in hydrogel formed compact aggregates that were distinct from each other (Fig. 1H,J,L).
Fig. 1. Representative phase contrast microscopy images of CCF-STTG1 and LN-18 cells maintained in monolayer and hydrogel culture systems.
CCF-STTG1 (A,C,E) and LN-18 (G,I,K) cells cultured on tissue culture polystyrene spread horizontally and appeared phase-dark. When cultured in hydrogel, CCF-STTG1 cells (B,D,F) formed regions of aggregated cells that were interconnected by regions of sparser cell density. LN-18 cells (H,J,L) formed defined and distinctive clusters. The images shown in the figure were collected from independent experiments conducted with cells subcultured from different frozen stock sister ampules of CCF-STTG1 (A,B: Ampule 1; C,D: Ampule 2; E,F: Ampule 3) and LN-18 (G,H: Ampule 1; I,J: Ampule 2; K,L: Ampule 3). Magnification: 20X. Scale bar = 50 μm
Epifluorescence microscopy was used as an additional tool to characterize the contrasting morphologies of the two culture systems following labelling with fluorescent probes for F-actin (Alexa Fluor® 488 Phalloidin) and DNA (Hoechst 33342) (Fig. 2). In monolayer culture systems, CCF-STTG1 cells appeared flat and spread horizontally on rigid tissue culture surfaces. The traditional culture system promoted cell growth, adjacent to each other without clumping or multilayer growth. Irregular shaped nuclei, some sickle shaped, were visible in both culture systems. Hydrogel cultures of CCF-STTG1 enabled the cells to grow in clusters with strands of cells bridging the masses (Fig. 2D,E,F), while LN-18 cells grew in the form of aggregates that were distinct from each other (Fig. 2J,K,L). Color epifluorescence images are depicted in Online Resource 1.
Fig. 2. Epifluorescence microscopy of CCF-STTG1 and LN-18 cells maintained in monolayer and hydrogel culture systems.
Monolayer and hydrogel cultures of CCF-STTG1 and LN-18 cells were imaged using fluorescent probes for F-actin and the nucleus. Monolayer CCF-STTG1 (Panels A-C) and LN-18 (Panels G-I) cells grew as stellar or oblong shapes and extended on the surface of the tissue culture slide. Hydrogels elicited the formation of aggregated multicellular structures in both cell lines. In CCF-STTG1 cultures (Panels D-F), strands of cells bridged the regions between the masses, while LN-18 cells showed formation of more compact clusters (Panels J-L). C,F,I,L: Alexa Fluor®488 Phalloidin (F-actin, green); B,E,H,K: Hoechst 33342 (DNA, blue); A,D,G,J: Merge. Scale bar = 50 μm. See Online Resource 1 for color images
3.2 Quantification of fluorescence images revealed a higher number of nuclei in hydrogel cultures than those in monolayer cultures of astrocytoma
Analysis of the number of nuclei present in monolayer and hydrogel cultures of astrocytoma showed that the hydrogel cultures had approximately twice the number of nuclei (as compared to their monolayer counterparts (P value < 0.05) within a comparable area, suggesting that the hydrogel may stimulate cell proliferation. The data were shown as the number of nuclei per 100 μm x 100 μm area in Online Resource 2. The area of the non-fluorescent background was found to be similar for the monolayer and hydrogel cultures of CCF-STTG1 and LN-18 cells (P value > 0.05; Online Resource 2).
3.3 Ultrastructural analysis with TEM uncovers autophagic vacuoles and nuclear membrane invaginations
TEM imaging of monolayer and hydrogel cultures uncovered ultrastructural features of CCF-STTG1 cells (Fig. 3). The cells in monolayer environments (Fig. 3A,B,C) exhibited elongated nuclei (Fig. 3A,‘n’) and a few autophagic vacuoles (Fig. 3A,B,C, white arrowheads). Intercellular junctions were present between neighboring cells (Fig. 3B, white arrows). The cells cultured in hydrogel (Fig. 3D,E,F) comprised nuclei (Fig. 3D,E,‘n’), intercellular junctions (Fig. 3F, white arrows) and autophagic vacuoles (Fig. 3D,E, white arrowheads). Nuclear membrane invaginations were present in cells cultured under both conditions (Fig. 3A,F, black arrows). The Geltrex™ matrix could be observed around cells in hydrogel as a grainy material indicating migration of cells into the hydrogel (Fig. 3F).
3.4 Autophagy-linked genes were identified by analysis of open source data sets
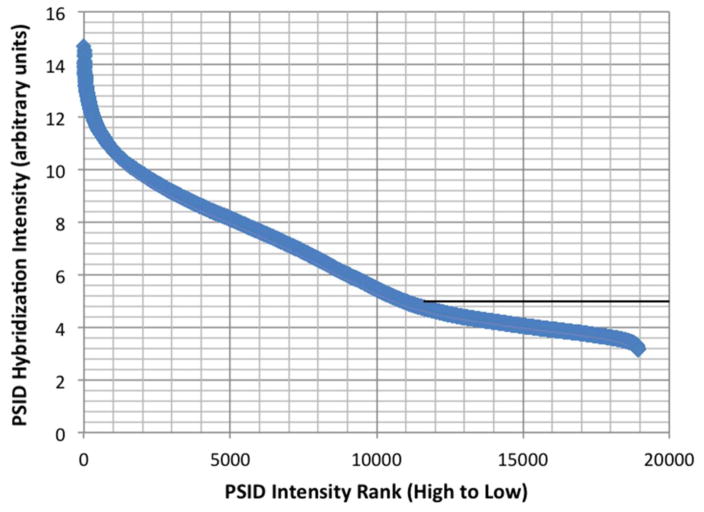
Microarray GEO DataSets for the CCF-STTG1 cell line (Online Resource 3) and the normal astrocyte cell line (Online Resource 4) were mined for expression of autophagy genes. The microarray dataset for CCF-STTG1 comprised intensity values ranging from 3.2 to 14.7 arbitrary units of fluorescence (AU; 8.0 ± 2.6; mean ± S.D.). The ‘Present/Marginal/Absent’ calls for both cell lines were retrieved by submitting Affymetrix CEL files to the Array Analysis website [26](Online Resource 3, 4). The intensity values for the probe set IDs (PSIDs) with ‘Present/Marginal/Absent’ calls were graphed as a scatter plot from highest to lowest intensity for the CCF-STTG1 cell line (Fig. 4; Online Resource 3). ‘Absent’ calls comprised 41% of the PSIDs and are predominant at intensity values less than 5.5 as indicated by the line in Fig. 4. We also compared the ‘Present/Marginal/Absent’ calls for the 222 HADb autophagy pathway genes with corresponding PSIDs in the CCF-STTG1 and normal astrocyte microarray data (Online Resource 3, 4). Analysis uncovered ‘Present/Marginal’ expression of 170 of the 222 autophagy genes in all microarray datasets for both cell lines. Eleven HADb genes were ‘Present’ only in CCF-STTG1, and nine genes only in normal astrocytes, while thirty HADb genes were assigned ‘Absent’ or ‘No P/M/A Base Call (NBC)’ (Online Resource 5).
Fig. 4. Scatterplot of microarray intensity values from CCF-STTG1 GEO DataSet GSM886923.
Gene Probe Set Identifiers (PSIDs) with ‘Present/Marginal/Absent’ were rank ordered from highest to lowest intensity value along the abscissa. The horizontal line highlights the region of intensity values where ‘Absent’ calls were predominant. (Online Resource 3)
3.5 Ontological and pathway analyses uncovered functional significance of HADb gene lists
Ontological analyses and functional annotation clustering were undertaken for the 181 HADb genes corresponding to PSIDs with a ‘Present’ call in the CCF-STTG1 microarray data using the default GO categories ‘biological process’, ‘cellular component’ and ‘molecular function’. GO reported 448 terms (biological process, 307; cellular component, 72; molecular function, 69) that were significantly associated with the HADb genes (P value < 0.05; Online Resource 6). Functional annotation clustering for these genes resulted in 39 significant clusters (enrichment score > 1.3, P value < 0.05) as summarized in Online Resource 7.
KEGG pathway analysis was undertaken for the 181 HADb genes with PSIDs assigned a ‘Present’ call in the CCF-STTG1 microarray data (Online Resource 5). Our results show that 132 of the 181 HADb genes are significantly involved in 84 different pathways (P value < 0.05). The KEGG pathways for the ‘Regulation of autophagy’ and ‘Pathways in cancer (Glioma)’ are graphically summarized in Fig. 5 and Fig. 6 respectively. The regulation of autophagy pathway depicts several interconnected signaling events occurring within the cell during various stages of autophagy such as induction, nucleation of autophagosomes, and their elongation and maturation (Fig. 5). Fig. 6 highlights the complex interplay between several genes involved in two alternate pathways in glial cells that lead to the formation of GBMs, namely de-novo pathway and secondary pathway.
3.6 BECN1 and LC3B gene expression is lower in monolayer cultures than in hydrogel cultures
BECN1 and LC3B were chosen for gene expression analysis because they are key autophagy biomarkers [33, 34]. Moreover, these genes had intermediate intensity values (~9 A.U.; max, 14.6) in microarray expression data for the CCF-STTG1 cell line (Online Resource 3), indicating they were abundant targets and good prospects for gene amplification. The RNA was isolated from the monolayer and hydrogel cultures of CCF-STTG1 and the quality of RNA was assessed using Agilent Bioanalyzer 2100. RIN values were not displayed by the Agilent software due to the low RNA concentration of RNA [35]. However, upon visual inspection of the electropherograms (Online Resource 8, Fig. 1) the expected number of peaks and low baseline between peaks were observed indicating that the RNA was intact. The Agilent chip electrophoresis of RNA samples showed crisp bands and no degradation products were observed (Online Resource 8, Fig. 2). The concentrations of RNA samples were determined (Online Resource 8 Table 1) and cDNA was prepared using the same RNA and its concentration was measured using Nanodrop Lite Spectrophotometer (Online Resource 8, Table 2). The quality of cDNA prepared from monolayer and hydrogel RNA samples was assessed with polymerase chain reaction (PCR) using primers for BECN1, LC3B, ACTB, and GAPDH genes (Methods, Table 1). Agarose gel electrophoresis results are shown in Online Resource 8.
qPCR plate reactions were completed with RNA isolated from hydrogel and monolayer cultures prepared from three different stock vials of CCF-STTG1 cells. Raw CT values for all the qPCR plates, PCR efficiency, R2 values and slopes for the standard curves are shown in Online Resource 8. Expression fold change values for each replicate are shown in (Table 2) for the hydrogel treatment group as compared to the monolayer calibrator group.
The BECN1 gene showed 2-fold and 1.5-fold higher relative expression in hydrogel cultures as compared to monolayer cultures, when normalized to ACTB and GAPDH respectively. The LC3B gene showed 3.9-fold and 1.7-fold higher relative expression in hydrogel cultures than in monolayer cultures, when normalized to ACTB and GAPDH respectively. Our findings were found to be significant for BECN1 when normalized to ACTB and for LC3B when normalized to ACTB and GAPDH (P value < 0.05). The greater variation in raw CT values for the GAPDH gene between the monolayer and hydrogel conditions may explain differences in the outcomes between the two reference genes.
3.7 The number of articles published using female donor glioblastoma cell lines is lower than those published using male donor cell lines
Analysis of the glioblastoma cell lines provided by ATCC revealed that 3 (27%) of 11 ATCC cell lines are available from female donors. Moreover, the number of articles published using female donor cell lines in the past 10 years is lower than those using male donor cell lines, comprising 9% of the total number of published articles (P-value < 0.05, Online Resource 9).
4. DISCUSSION
When CCF-STTG1 and LN-18 cells were grown in conventional monolayer cultures, they appeared phase-dark, flattened, and were dispersed over the slide surface. In contrast, CCF-STTG1 cells cultured in Geltrex matrix aggregated into clusters with interconnecting strands of cells. Images acquired with TEM (Fig. 3) suggest that CCF-STTG1 cells migrate into the Geltrex matrix. Previous studies have shown that the migration and morphology of glioblastoma cells are affected by the type and concentration of hydrogel used for cell culture [36]. Analysis of the number of nuclei present in monolayer and hydrogel cultures of astrocytoma showed that twice as many nuclei were present in hydrogel cultures of comparable area (Online Resource 2), suggesting that cells may proliferate faster in hydrogel than monolayer environments [37]. The LN-18 cells also formed compact distinctive spheroids in hydrogel. Interestingly, previous studies have demonstrated the formation of neurospheres by LN-18 cells in the presence of growth factors [38]. These combined results are consistent with research that shows that the morphology of cells in culture is responsive to hydrogel environments [8, 9] and warrant future experiments examining the impact of serum-free media on cell growth in monolayer and hydrogel cultures [38, 39].
Studies that examine cellular features of astrocytoma emphasize light microscopy methods and in comparison, far less is known about their high resolution cellular ultrastructure [40, 41]. The scarcity of ultrastructural information prompted our efforts to examine features of monolayer and hydrogel CCF-STTG1 cultures with TEM. Nuclear membrane invaginations are considered a hallmark of cancer cells [42–44] and TEM images of astrocytoma cells in monolayer and in hydrogel cultures exhibited nuclear membrane invaginations similar to those observed in breast cancer, thyroid cancer, acute myeloid leukemia and other gliomas [42–44]. These invaginations are thought to be linked to the ability of cancer cells to migrate to distant locations via metastasis and altered calcium signalling in these cells [44, 45]. In addition, the formation of these invaginations has been attributed to a change in nuclear lamins, which in turn regulate the rigidity and shape of the nucleus [45]. Images of cells labelled with the fluorescent DNA probe Hoechst 33342 showed elongated and irregular sickle-shaped nuclei in both monolayer and hydrogel cultures (Fig. 2). These results are in agreement with previous work conducted on high-grade gliomas in mouse brains, where they have reported the presence of spindle-shaped cells containing elongated nuclei [46].
Autophagic vacuoles were visible in ultrastructural images of monolayer and hydrogel cultures (Fig 3). Among many physiological roles, autophagy aids in maintaining homeostasis and has been implicated in starvation adaptation, cell death, tumor suppression, antigen presentation, development, and clearance of intracellular proteins and organelles [47–51]. Autophagy is also involved in different pathologies such as cancer, inflammation, and neurodegenerative conditions [52]. In cancer, autophagy has a complex dual role as it can assist in suppressing tumor growth and also promote tumor cell survival [53–57].
We were intrigued by the observation of autophagic vacuoles in CCF-STTG1 cells and sought to probe genetic underpinnings by analyzing available datasets. We began by searching for open source transcriptome profiling data that could facilitate in silico analysis. Queries of the NCBI GEO DataSet repository retrieved microarray datasets for CCF-STTG1 and a normal human astrocyte cell line. When we cross-referenced the microarray expression data with the autophagy-linked genes listed on HADb, we noted that 170 of the 222 autophagy genes were assigned ‘Present/Marginal’ calls in both cell lines. Some HADb genes were assigned ‘Present/Marginal’ calls in only one of the cell lines (CCF-STTG1, eleven; normal astrocytes, nine), while thirty HADb genes were assigned ‘Absent’ or ‘No P/M/A Base Call (NBC)’ (Online Resource 5). Ontological analyses of 181 HADb genes corresponding to PSIDs with a ‘Present’ call in the CCF-STTG1 microarray data yielded 448 GO terms associated with these genes (Online Resource 6). KEGG pathway analysis showed that 77% of the 181 HADb genes were involved in 84 different pathways including those associated with autophagy (Fig. 5) and glioma (Fig. 6).
The intensity values from the microarray dataset informed pilot efforts to examine potential differences in gene expression elicited by the two culture environments. Microarray data intensity values are correlated with transcript abundance and directed our experimental efforts toward qPCR analysis of BECN1 and LC3B gene expression in monolayer and hydrogel cultures because both genes had intermediate intensity values of approximately 9 (Online Resource 3). BECN1 has been identified as a key autophagy regulator protein involved in the formation, extension and maturation of autophagosome, a unique organelle which mediates autophagy phenomenon [58–60], while LC3B is one of the structural proteins involved in the formation of the autophagosomal membrane and is a biomarker routinely used for monitoring autophagy [33]. At least two reference genes are recommended to improve reliability and accuracy of qPCR normalization [61–63]. Accordingly, we selected ACTB and GAPDH as reference genes due to their high expression levels in the cell cultures [64]. Our qPCR analysis showed a modest relative increase in the expression of BECN1 and LC3B genes in hydrogel cultures as compared to monolayer cultures. Changes in BECN1 and LC3B expression have been reported as features of the cancer state. Some findings suggest that BECN1 is upregulated in glioma and colorectal cancers, leading to poor prognosis in patients [65–67], while others observe a reduction in BECN1 and LC3B expression, leading to the tumor progression in gliomas and other cancer types [34, 68–71]. These differences may underscore the complexity of the role of autophagy in cancer progression as well as differences in tissue type and origin [23, 72].
Finally, in the process of selecting the CCF-STTG1 cell line for experimentation, we noted that although there is much evidence in support of sex differences in brain cancer incidence, disease progression, and response to treatment, fewer studies have been undertaken with commercial astrocytoma cell lines from female donors. For example, we observed that 27% of the ATCC collection of adult human glioblastoma cell lines that includes CCF-STTG1 were of female origin. Furthermore, queries of PUBMED showed that in the past 10 years, only 9% of the publications with the ATCC human glioblastoma cell lines have incorporated cell lines from female donors (Online Resource 9). We sought to bridge this knowledge gap by undertaking experiments with the female line, CCF-STTG1. The literature demonstrating sex-based differences in astrocytoma progression, therapeutic outcomes and mortality, supports greater consideration of this variable in the use of human cell lines for gliomas [2, 22, 73].
Summary
Our findings show that hydrogels such as Geltrex™ can be readily adapted for astrocytoma culture systems and reinforce the idea that the tissue microenvironment plays a key role in modulating cellular morphology and gene expression [8, 9]. Our results demonstrate the presence of autophagic vacuoles and nuclear membrane invaginations in a high-grade astrocytoma culture, and suggest that the autophagy biomarkers, BECN1 and LC3B, may be elevated in hydrogel environments. Methods presented here can be useful for other investigators who seek protocols for hydrogel culture and for preparing tissue culture samples for transmission electron microscopy, as well as those who seek to include a female astrocytoma cell line in their experimental design. Taken together, our findings suggest that autophagy may play a role in growth and survival of CCF-STTG1 cells and that autophagy genes and their products may be potential targets for intervention strategies against astrocytomas.
Supplementary Material
This file shows images of monolayer and hydrogel cultures of CCF-STTG1 and LN-18 cells captured using epifluorescence microscopy and fluorescent probes- Hoechst 33342 (DNA) and Alexa Fluor® 488 phalloidin (F-actin). This color image of Figure 1 was provided for the reader’s reference.
This file comprises the data and analysis used to determine 1) the number of nuclei and 2) the areas in the dark background, from images of monolayer and hydrogel cultures of CCF-STTG1 and LN-18.
This file reports the Affymetrix ‘Present/Marginal/Absent’ calls retrieved from CEL files for GEO DataSet GSM886923 for the CCF-STTG1 cell line submitted to the Array Analysis website are along with their PSIDs.
Online Resource 4. The ‘Present/Marginal/Absent’ Calls for PSIDs from GEO DataSets GSM397656, GSM397657 and GSM397658 for the Normal Human Astrocyte Cell Line. This file reports the Ensembl Gene IDs and their 'Present/Marginal/Absent' calls retrieved from CEL files using the Array Analysis website for GEO DataSets GSM397656, GSM397657 and GSM397658 for normal human astrocytes.
Data shown here compare the expression of autophagy genes in CCF-STTG1 cells and normal human astrocytes.
This file summarizes the outcomes of DAVID ontological analysis of 181 HADb genes identified in CCF-STTG1 microarray data
This file summarizes the outcomes of functional annotation clustering and KEGG pathway analysis conducted using DAVID for 181 HADb genes identified in CCF-STTG1 microarray data.
This file shows the data for quality assessment of RNA and cDNA and the data for qPCR analysis.
This file shows the name and donor gender for ATCC glioblastoma cell lines and the number of publications found in PUBMED for each cell line during the past 10 years.
Acknowledgments
Funding. This research was supported by the New Mexico State University Manasse Chair Endowment, the National Institutes of Health (P50GM68762) and the National Science Foundation (MRI-DBI-0520956).
We would like to thank Dr. Peter Cooke for technical assistance and access to the Electron Microscope facility in the Core University Research Resources Laboratory (CURRL) at New Mexico State University.
Abbreviations
- 2D
two-dimensional
- 3D
three-dimensional
- AU
arbitrary units
- BECN1
Beclin 1
- bp
base pair
- cDNA
complementary deoxyribonucleic acid
- CNS
central nervous system
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- dH2O
distilled water
- DNA
deoxyribonucleic acid
- GBM
glioblastoma
- GEO
Gene Expression Omnibus
- GO
Gene Ontology
- HADb
human autophagy database
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LC3B
microtubule-associated proteins 1A/1B light chain 3B
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PSID
probe set ID
- qPCR
quantitative PCR
- RMA
robust multi array average
- RNA
ribonucleic acid
- S.E.
standard error
- STR
short tandem repeat
- TEM
transmission electron microscopy
Footnotes
Conflict of Interest. The authors declare that they have no conflict of interest. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Science Foundation or New Mexico State University.
Electronic supplementary material
The online version of this article (doi:TBA) contains supplementary material, which is available to authorized users.
References
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2005–2009. Neuro Oncol. 2012 Nov;14(suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015 Sep;72(17):3323–42. doi: 10.1007/s00018-015-1930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G. Long-term survival with glioblastoma multiforme. Brain. 2007 Oct;130(Pt 10):2596–606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 4.Astashkina A, Mann B, Grainger DW. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther. 2012 Apr;134(1):82–106. doi: 10.1016/j.pharmthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Reardon DA, Rich JN, Friedman HS, Bigner DD. Recent Advances in the Treatment of Malignant Astrocytoma. J Clin Oncol. 2006 Mar;24(8):1253–1265. doi: 10.1200/JCO.2005.04.5302. [DOI] [PubMed] [Google Scholar]
- 6.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012 Jul;125(Pt 13):3015–24. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014 May;12(4):207–18. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werfel J, Krause S, Bischof AG, Mannix RJ, Tobin H, Bar-Yam Y, Bellin RM, Ingber DE. How Changes in Extracellular Matrix Mechanics and Gene Expression Variability Might Combine to Drive Cancer Progression. PLoS One. 2013 Oct;8(10):e76122. doi: 10.1371/journal.pone.0076122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovitt CJ, Shelper TB, Avery VM. Advanced cell culture techniques for cancer drug discovery. Biology (Basel) 2014 Jan;3(2):345–67. doi: 10.3390/biology3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess MW, Pfaller K, Ebner HL, Beer B, Hekl D, Seppi T. Electron Microscopy of Model Systems. Vol. 96. Elsevier; 2010. [DOI] [PubMed] [Google Scholar]
- 11.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006 Oct;3(10):589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob Cardiol Sci Pract. 2013;2013(3):316–42. doi: 10.5339/gcsp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009 Jul;103(4):655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science (80- ) 2017;356(6337) doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann D, Conway JRW, Vennin C, Magenau A, Hughes WG, Morton JP, Timpson P. Three-dimensional cancer models mimic cell–matrix interactions in the tumour microenvironment. 2014 doi: 10.1093/carcin/bgu108. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. 2014 doi: 10.1016/j.biotechadv.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R, Bevilacqua A, Tesei A, Fricker J, Ocana A, Pandiella A, Siu LL, Tannock IF, Sams-Dodd F, Edwards AM, Lee GY, Kenny PA, Lee EH, Bissell MJ, Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W, Kimlin LC, Casagrande G, Virador VM, Baker BM, Chen CS, Wartenberg M, Minchinton AI, Tannock IF, Weiswald LB, Bellet D, Dangles-Marie V, Yamada KM, Cukierman E, Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA, Jaganathan H, Cunha C, Panseri S, Villa O, Silva D, Gelain F, Tesei A, Vinci M, Melo FDSE, Vermeulen L, Fessler E, Medema JP, Mueller-Klieser W, Mueller-Klieser W, Mueller-Klieser W, Hirschhaeuser F, Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S, Frankel A, Buckman R, Kerbel RS, Dubessy C, Kim TH, Mount CW, Gombotz WR, Pun SH, Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G, Celli JP, Piccinini F, Piccinini F, Tesei A, Arienti C, Bevilacqua A, Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK, Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH, Pignatta S, Grimm D, Ingram M, Dufau I, Sorensen AG, Waschow M, Letzsch S, Boettcher K, Kelm J, Hirschhaeuser F, Walenta S, Mueller-Klieser W, Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU, Haisler WL, Piccinini F, Tesei A, Paganelli G, Zoli W, Bevilacqua A. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep. 2016 Jan;6:19103. doi: 10.1038/srep19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Fan X, Houghton J. Tumor microenvironment: The role of the tumor stroma in cancer. J Cell Biochem. 2007 Jul;101(4):805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 19.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008 Oct;27(45):5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Sabanayagam CR, Harrington DA, Farach-Carson MC, Jia X. A hydrogel-based tumor model for the evaluation of nanoparticle-based cancer therapeutics. Biomaterials. 2014 Mar;35(10):3319–30. doi: 10.1016/j.biomaterials.2013.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. [Accessed: 10-Apr-2015];CCF-STTG1 ATCC ® CRL-1718™ Homo sapiens brain grade IV, ast. [Online]. Available: http://www.atcc.org/products/all/CRL-1718.aspx.
- 22.Verger E, Valduvieco I, Caral L, Pujol T, Ribalta T, Viñolas N, Boget T, Oleaga L, Blanco Y, Graus F. Does gender matter in glioblastoma? Clin Transl Oncol. 2011 Oct;13(10):737–741. doi: 10.1007/s12094-011-0725-7. [DOI] [PubMed] [Google Scholar]
- 23.Ciechomska IA, Gabrusiewicz K, Szczepankiewicz AA, Kaminska B. Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine A-induced cell death. Oncogene. 2013 Mar;32(12):1518–1529. doi: 10.1038/onc.2012.174. [DOI] [PubMed] [Google Scholar]
- 24. [Accessed: 06-Jan-2017];Geltrex LDEV-Free Reduced Growth Factor Basement Membrane Matrix - Thermo Fisher Scientific. [Online]. Available: https://www.thermofisher.com/order/catalog/product/A1413202.
- 25.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012 Jul;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eijssen LMT, Jaillard M, Adriaens ME, Gaj S, de Groot PJ, Muller M, Evelo CT. User-friendly solutions for microarray quality control and pre-processing on ArrayAnalysis.org. Nucleic Acids Res. 2013 Jul;41(Web Server issue):W71–6. doi: 10.1093/nar/gkt293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009 Jan;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 28.Guide to Performing Relative Quantitation of Gene Expression Using Real-Time Quantitative PCR
- 29.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2 ΔΔCT Method. Methods. 2001 Dec;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Atcc.org. [Accessed: 11- May- 2017];Brain Tumor Cell Lines'. 2011 [Online]. Available: https://www.atcc.org/~/media/PDFs/Brain_Tumor_Cell_Lines.ashx.
- 31.Barna BP, Chou SM, Jacobs B, Ransohoff RM, Hahn JF, Bay JW. Enhanced DNA synthesis of human glial cells exposed to human leukocyte products. J Neuroimmunol. 1985 Dec;10(2):151–8. doi: 10.1016/0165-5728(85)90005-0. [DOI] [PubMed] [Google Scholar]
- 32.Diserens AC, de Tribolet N, Martin-Achard A, Gaide AC, Schnegg JF, Carrel S. Characterization of an established human malignant glioma cell line: LN-18. Acta Neuropathol. 1981;53(1):21–8. doi: 10.1007/BF00697180. [DOI] [PubMed] [Google Scholar]
- 33.Koukourakis MI, Kalamida D, Giatromanolaki A, Zois CE, Sivridis E, Pouliliou S, Mitrakas A, Gatter KC, Harris AL. Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines. PLoS One. 2015 Sep;10(9):e0137675. doi: 10.1371/journal.pone.0137675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tini P, Belmonte G, Toscano M, Miracco C, Palumbo S, Pastina P, Battaglia G, Nardone V, Butorano MAGM, Masucci A, Cerase A, Pirtoli L. Combined epidermal growth factor receptor and Beclin1 autophagic protein expression analysis identifies different clinical presentations, responses to chemo- and radiotherapy, and prognosis in glioblastoma. Biomed Res Int. 2015;2015:208076. doi: 10.1155/2015/208076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller O, Lightfoot S, Schroeder A. RNA Integrity Number (RIN) – Standardization of RNA Quality Control Application [Google Scholar]
- 36.Rao SS, DeJesus J, Short AR, Otero JJ, Sarkar A, Winter JO. Glioblastoma Behaviors in Three-Dimensional Collagen-Hyaluronan Composite Hydrogels. ACS Appl Mater Interfaces. 2013 Oct;5(19):9276–9284. doi: 10.1021/am402097j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lei Y, Gojgini S, Lam J, Segura T. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials. 2011 Jan;32(1):39–47. doi: 10.1016/j.biomaterials.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciechomska IA, Przanowski P, Jackl J, Wojtas B, Kaminska B. BIX01294, an inhibitor of histone methyltransferase, induces autophagy-dependent differentiation of glioma stem-like cells. Sci Rep. 2016 Dec;6(1):38723. doi: 10.1038/srep38723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rasper M, Schafer A, Piontek G, Teufel J, Brockhoff G, Ringel F, Heindl S, Zimmer C, Schlegel J. Aldehyde dehydrogenase 1 positive glioblastoma cells show brain tumor stem cell capacity. Neuro Oncol. 2010 Oct;12(10):1024–1033. doi: 10.1093/neuonc/noq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu R, Li J, Zhang T, Zou L, Chen Y, Wang K, Lei Y, Yuan K, Li Y, Lan J, Cheng L, Xie N, Xiang R, Nice EC, Huang C, Wei Y. Itraconazole suppresses the growth of glioblastoma through induction of autophagy. Autophagy. 2014 Jul;10(7):1241–1255. doi: 10.4161/auto.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryskalin L, Lenzi P, Falleni A, Guagnozzi M, Paparelli S, Bartalucci A, Flaibani M, Fornai F. Ultrastructure of Glioblastoma cells in baseline conditions and following mTOR inhibtion. Ital J Anat Embryol. 2014;119(1):171. [Google Scholar]
- 42.Chen W, Konoplev S, Medeiros LJ, Koeppen H, Leventaki V, Vadhan-Raj S, Jones D, Kantarjian HM, Falini B, Bueso-Ramos CE. Cuplike nuclei (prominent nuclear invaginations) in acute myeloid leukemia are highly associated with FLT3 internal tandem duplication and NPM1 mutation. Cancer. 2009 Dec;115(23):5481–5489. doi: 10.1002/cncr.24610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terés S, Lladó V, Higuera M, Barceló-Coblijn G, Martin ML, Noguera-Salvà MA, Marcilla-Etxenike A, García-Verdugo JM, Soriano-Navarro M, Saus C, Gómez-Pinedo U, Busquets X, Escriba PV. 2-Hydroxyoleate, a nontoxic membrane binding anticancer drug, induces glioma cell differentiation and autophagy. Proc Natl Acad Sci U S A. 2012 May;109(22):8489–94. doi: 10.1073/pnas.1118349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow KH, Factor RE, Ullman KS. The nuclear envelope environment and its cancer connections. Nat Rev Cancer. 2012 Feb;12(3):196–209. doi: 10.1038/nrc3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011 Feb;23(1):55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghazi SO, Stark M, Zhao Z, Mobley BC, Munden A, Hover L, Abel TW. Cell of origin determines tumor phenotype in an oncogenic Ras/p53 knockout transgenic model of high-grade glioma. J Neuropathol Exp Neurol. 2012 Aug;71(8):729–40. doi: 10.1097/NEN.0b013e3182625c02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB., III Autophagy: regulation and role in development. Autophagy. 2013 Jul;9(7):951–72. doi: 10.4161/auto.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang G, Mao Z. Chaperone-mediated autophagy: roles in neurodegeneration. Transl Neurodegener. 2014;3:20. doi: 10.1186/2047-9158-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai Z, Zeng W, Tao K, Zhen E, Wang B, Yang Q. Chaperone-mediated autophagy: roles in neuroprotection. Neurosci Bull. 2015 Aug;31(4):452–458. doi: 10.1007/s12264-015-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014 Jan;24(1):92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jing K, Lim K. Why is autophagy important in human diseases? Exp Mol Med. 2012 Feb;44(2):69. doi: 10.3858/emm.2012.44.2.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galluzzi L, Kroemer G. Defective autophagy gets to the brain. Oncotarget. 2015 Nov;6(37):39396–7. doi: 10.18632/oncotarget.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang YH, Al-Aidaroos AQO, Yuen HF, Zhang SD, Shen HM, Rozycka E, McCrudden CM, Tergaonkar V, Gupta A, Bin Lin Y, Thiery JP, Murray JT, Zeng Q. A role of autophagy in PTP4A3-driven cancer progression. Autophagy. 2014 Oct;10(10):1787–800. doi: 10.4161/auto.29989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan MJ, Gamez G, Menke C, Hernandez A, Thorburn J, Gidan F, Staskiewicz L, Morgan S, Cummings C, Maycotte P, Thorburn A. Regulation of autophagy and chloroquine sensitivity by oncogenic RAS in vitro is context-dependent. Autophagy. 2014 Oct;10(10):1814–26. doi: 10.4161/auto.32135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaminskyy VO, Piskunova T, Zborovskaya IB, Tchevkina EM, Zhivotovsky B. Suppression of basal autophagy reduces lung cancer cell proliferation and enhances caspase-dependent and -independent apoptosis by stimulating ROS formation. Autophagy. 2012 Jul;8(7):1032–44. doi: 10.4161/auto.20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mah LY, Ryan KM. Autophagy and cancer. Cold Spring Harb Perspect Biol. 2012 Jan;4(1):a008821. doi: 10.1101/cshperspect.a008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, Kimmelman A, Kumar S, Levine B, Maiuri MC, Martin SJ, Penninger J, Piacentini M, Rubinsztein DC, Simon H-U, Simonsen A, Thorburn AM, Velasco G, Ryan KM, Kroemer G. Autophagy in malignant transformation and cancer progression. EMBO J. 2015 Apr;34(7):856–80. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008 Jan;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008 Feb;451(7182):1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011 Apr;18(4):571–80. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koepke JF, Koepke JA, Jiang J, Thein SL, Koepke J, Koepke J, Dheda K, Huggett J, Bustin S, Johnson M, Rook G, Zumla A, Karge W, Schaefer E, Ordovas J, Thellin O, Zorzi W, Lakaye B, De B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E, Suzuki T, Higgins P, Crawford D, Deindl E, Boengler K, van R, Schaper W, Hamalainen H, Tubman J, Vikman S, Kyrola T, Ylikoski E, Warrington J, Lahesmaa R, Zhong H, Simons J, Glare E, Divjak M, Bailey M, Walters E, Radonic A, Thulke S, Mackay I, Landt O, Siegert W, Nitsche A, Vandesompele J, De P, Pattyn F, Poppe B, Van R, De P, Speleman F, Pfaffl M, Tichopad A, Prgomet C, Neuvians T, Andersen C, Jensen J, Orntoft T, Dydensborg A, Herring E, Auclair J, Tremblay E, Beaulieu J, Walker N, Haberhausen G, Pinsl J, Kuhn C, Markert-Hahn C, Zhang X, Ding L, Sandford A, Bonafoux B, Lejeune M, Piquemal D, Quere R, Baudet A, Assaf L, Marti J, guilar-Martinez P, Commes T, Spector T, Macgregor A, Pal S, Nemeth M, Bodine D, Miller J, Svaren J, Thein S, Lowry P, Bresnick E, Haverty P, Weng Z, Best N, Auerbach K, Hsiao L, Jensen R, Gullans S. Reticulocytes. Clin Lab Haematol. 1986 Sep;8(3):169–179. doi: 10.1111/j.1365-2257.1986.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 62.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002 Jun;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petriccione M, Mastrobuoni F, Zampella L, Scortichini M, Wise RP, Moscou MJ, Bogdanove AJ, Whitham SA, Pfaffl MW, Dorak T, Kundu S, Chakraborty D, Kundu A, Pal A, Bustin SA, Guenin S, Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR, Huggett J, Dheda K, Bustin S, Zumla A, Imai T, Ubi BE, Saito T, Moriguchi T, Llanos A, François JM, Parrou JL, Willems E, Leyns L, Vandesompele J, Adomas A, Rinaldi C, Figueiredo A, Jarosová J, Kundu JK, Mascia T, Santovito E, Gallitelli D, Cillo F, Monteiro F, Sebastiana M, Pais MS, Figueiredo A, Jacob TR, Laia ML, Ferro JA, Ferro MIT, Mafra V, Takle GW, Toth IK, Brurberg MB, Scortichini M, Marcelletti S, Ferrante P, Petriccione M, Firrao G, Marcelletti S, Ferrante P, Petriccione M, Firrao G, Scortichini M, Mazzaglia A, McCann HC, Ferrante P, Scortichini M, Petriccione M, Di Cecco I, Arena S, Scaloni A, Scortichini M, Petriccione M, Salzano AM, Di Cecco I, Scaloni A, Scortichini M, Vandesompele J, Andersen CL, Jensen JL, Orntoft TF, Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP, Silver N, Best S, Jiang J, Thein SL, Walton EF, Ledger SE, Janssen BJ, Karunairetnam S, Wang T, Snowden KC, Nardozza S, Gunther CS, Chervin C, Marsh KB, Newcomb RD, Souleyre EJF, Selim M, Ebadzad G, Cravador A, Matsumura H, Nirasawa S, Terauchi R, Gantasala N, Borges AF, Fonseca C, Ferreira RB, Lourenço AM, Monteiro S, Castro P, Roman B, Rubio J, Die J, Remans T, Robledo D, Lilly ST, Drummond RS, Pearson MN, Macdiarmid RM, Zhu J, Zhang L, Li W, Han S, Yang W, Qi L, Guo J, Ling H, Wu Q, Xu L, Que Y, Storch TT, Velada I, Ragonezi C, Arnholdt-Schmitt B, Cardoso H, Radonic A, Ramiro D, Borges A, Tsai S, Caldas D, Scholtz JJ, Visser B, Liu D, Wieczorek P, Wrzesińska B, Obrępalska-Stęplowska A, Fones HN, Preston GM, De Gara L, de Pinto MC, Tommasi F, Novogórska A, Patykovski J, Ferrante P, Scortichini M, Ferrante P, Scortichini M, Rubio-Pina JA, Zapata-Perez O, Radonić A, Livak KJ, Schmittgen TD. Reference gene selection for normalization of RT-qPCR gene expression data from Actinidia deliciosa leaves infected with Pseudomonas syringae pv. actinidiae. Sci Rep. 2015 Nov;5:16961. doi: 10.1038/srep16961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. [Accessed: 06-Jan-2017];GEO Accession viewer. [Online]. Available: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?view=data&acc=GSM886923&id=24002&db=GeoDb_blob74.
- 65.Koukourakis MI, Giatromanolaki A, Sivridis E, Pitiakoudis M, Gatter KC, Harris AL. Beclin 1 over- and underexpression in colorectal cancer: distinct patterns relate to prognosis and tumour hypoxia. Br J Cancer. 2010 Oct;103(8):1209–14. doi: 10.1038/sj.bjc.6605904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giatromanolaki A, Sivridis E, Mitrakas A, Kalamida D, Zois CE, Haider S, Piperidou C, Pappa A, Gatter KC, Harris AL, Koukourakis MI. Autophagy and lysosomal related protein expression patterns in human glioblastoma. Cancer Biol Ther. 2014;15(11):1468–78. doi: 10.4161/15384047.2014.955719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pirtoli L, Cevenini G, Tini P, Vannini M, Oliveri G, Marsili S, Mourmouras V, Rubino G, Miracco C. The prognostic role of Beclin 1 protein expression in high-grade gliomas. Autophagy. 2009 Oct;5(7):930–6. doi: 10.4161/auto.5.7.9227. [DOI] [PubMed] [Google Scholar]
- 68.Huang X, Bai HM, Chen L, Li B, Lu YC. Reduced expression of LC3B-II and Beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J Clin Neurosci. 2010 Dec;17(12):1515–1519. doi: 10.1016/j.jocn.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 69.Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, Mannucci S, De Nisi MC, Toscano M, Malagnino V, Falzarano SM, Pirtoli L, Tosi P. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol. 2007 Feb;30(2):429–36. [PubMed] [Google Scholar]
- 70.Li Z, Chen B, Wu Y, Jin F, Xia Y, Liu X. Genetic and epigenetic silencing of the beclin 1 gene in sporadic breast tumors. BMC Cancer. 2010 Mar;10:98. doi: 10.1186/1471-2407-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ying H, Qu D, Liu C, Ying T, Lv J, Jin S, Xu H. Chemoresistance is associated with Beclin-1 and PTEN expression in epithelial ovarian cancers. Oncol Lett. 2015 Apr;9(4):1759–1763. doi: 10.3892/ol.2015.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou Z, Yuan Z, Zhang Q, Long Z, Chen J, Tang Z, Zhu Y, Chen S, Xu J, Yan M, Wang J, Liu Q. Aurora kinase A inhibition-induced autophagy triggers drug resistance in breast cancer cells. Autophagy. 2012 Dec;8(12):1798–810. doi: 10.4161/auto.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holdcroft A. Gender bias in research: how does it affect evidence based medicine? J R Soc Med. 2007 Jan;100(1):2–3. doi: 10.1258/jrsm.100.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file shows images of monolayer and hydrogel cultures of CCF-STTG1 and LN-18 cells captured using epifluorescence microscopy and fluorescent probes- Hoechst 33342 (DNA) and Alexa Fluor® 488 phalloidin (F-actin). This color image of Figure 1 was provided for the reader’s reference.
This file comprises the data and analysis used to determine 1) the number of nuclei and 2) the areas in the dark background, from images of monolayer and hydrogel cultures of CCF-STTG1 and LN-18.
This file reports the Affymetrix ‘Present/Marginal/Absent’ calls retrieved from CEL files for GEO DataSet GSM886923 for the CCF-STTG1 cell line submitted to the Array Analysis website are along with their PSIDs.
Online Resource 4. The ‘Present/Marginal/Absent’ Calls for PSIDs from GEO DataSets GSM397656, GSM397657 and GSM397658 for the Normal Human Astrocyte Cell Line. This file reports the Ensembl Gene IDs and their 'Present/Marginal/Absent' calls retrieved from CEL files using the Array Analysis website for GEO DataSets GSM397656, GSM397657 and GSM397658 for normal human astrocytes.
Data shown here compare the expression of autophagy genes in CCF-STTG1 cells and normal human astrocytes.
This file summarizes the outcomes of DAVID ontological analysis of 181 HADb genes identified in CCF-STTG1 microarray data
This file summarizes the outcomes of functional annotation clustering and KEGG pathway analysis conducted using DAVID for 181 HADb genes identified in CCF-STTG1 microarray data.
This file shows the data for quality assessment of RNA and cDNA and the data for qPCR analysis.
This file shows the name and donor gender for ATCC glioblastoma cell lines and the number of publications found in PUBMED for each cell line during the past 10 years.