Abstract
Background:
Despite lack of effectiveness and potential harms, antipsychotic medications (APMs) are often prescribed off-label for postoperative delirium. We evaluated the temporal trends and between-hospital variation of off-label APM use in older cardiac surgical patients.
Design:
Retrospective cohort study.
Setting:
A national administrative database including 465 United States hospitals.
Participants:
293,212 patients ≥65 years, without known indications for APMs, who underwent cardiac surgery in 2004–2014.
Measurements:
Postoperative exposure to any APMs and potentially excessive dosing was examined. The hospital-level APM prescribing intensity was defined as the proportion of patients newly treated with APMs in the postoperative period.
Results:
The rate of APM use declined from 8.8% in 2004 to 6.2% in 2014 (p<0.001). Use of haloperidol (parenteral: 7.0% to 4.5%, p<0.001; oral: 1.9% to 0.5%, p<0.001) and risperidone (1.1% to 0.3%, p<0.001) declined, while quetiapine use tripled (0.6% to 1.9%, p=0.03). The hospital APM-prescribing intensity varied widely from 0.3% to 35.6% across 465 hospitals. Among the treated patients, those at higher prescribing hospitals were more likely to receive APMs on the day of discharge (highest vs lowest quintile: 15.1% vs 9.6%; p<0.001) and for a longer duration (4.8 vs 3.7 days; p<0.001). Delirium was the strongest risk factor for APM exposure (odds ratio, 9.73; 95% confidence interval, 9.02–10.5), whereas none of the hospital characteristics were significantly associated. The rate of potentially excessive dosing declined (60.7% to 44.9%, p<0.001) and the risk factors for potentially excessive dosing were similar to those for any APM exposure.
Conclusions:
Our findings suggest highly variable prescribing cultures and raise concerns about inappropriate use, highlighting the need for better evidence to guide APM prescribing in hospitalized older patients after cardiac surgery.
Keywords: Antipsychotics, Delirium, Cardiac Surgery
INTRODUCTION
Older adults undergoing cardiac surgery are at high risk for postoperative delirium.1–3 These patients often receive antipsychotic medications (APMs) off-label, despite clinical guidelines concerning the lack of effectiveness and serious harms.4–6 Clinical trials did not consistently demonstrate that APMs reduce delirium incidence, duration, or severity.7,8 Moreover, observational studies9–17 and clinical trials18,19 in older adults with dementia found that APMs increase the risk of sedation, extrapyramidal symptoms, cardiac arrhythmia, stroke, pneumonia, and even death. Older cardiac surgical patients are susceptible to these adverse events due to their cardiovascular disease and other comorbidities.20,21
To date, the characteristics of off-label APM prescribing have not been well examined in older patients after cardiac surgery. After the Centers for Medicare and Medicaid Services (CMS) began to measure the rate of APM use in nursing homes in 2012, the rate of off-label APM use declined from 24% in 2011 to 16% in 2016.22 In 2016, CMS proposed a similar metric for acute-care hospitals.23 Documenting the trends and patient and hospital characteristics associated with off-label APM use is an essential step to reduce excessive APM use in these patients.
This retrospective study was conducted to determine the longitudinal trends and variation of postoperative off-label APM use in a national database of nearly 300,000 older adults undergoing cardiac surgery at 465 United States (US) hospitals in 2004–2014. With the decline in off-label APM use in the nursing homes, we hypothesized that a similar downward trend would be observed in these patients.
METHODS
Data Source
The Premier Healthcare Database is an administrative dataset that contains billing and coding information on inpatients treated at over 700 hospitals, which account for 20% of all hospitalizations in the US.24 Demographic information, admission and discharge status, diagnoses, medications (including dosages), procedures, diagnostic tests, and hospital characteristics are recorded. We analyzed data from 2004–2014 to examine the temporal trend of APM use in cardiac surgical patients. Since this study involved analysis of de-identified data, the Brigham and Women’s Hospital Institutional Review Board determined that it qualified for human subject research exemption. A waiver for informed consent was granted.
Study Population
We included patients age 65 years or older who underwent coronary artery bypass grafting (International Classification of Diseases 9th revision [ICD-9] procedure codes 36.1x), valve surgery (ICD-9 procedure codes: 35.2x), or both. Patients with schizophrenic disorders (ICD-9 diagnosis codes: 295, V11.0), mood disorders (296), delusional disorders (297.1), non-organic psychoses (298), Tourette’s disorder (307.23), Huntington disease (333.4), hiccup (786.8), or chemotherapy (V58.1, V66.2, V67.2) were excluded. We also excluded patients treated with an APM before surgery to focus on the new postoperative APM use, and those treated on the day of surgery who might have received APMs for postoperative nausea and vomiting. Due to the possibility of incomplete reporting, we excluded data from hospitals that reported fewer than 20 cases in a given year.
APM Prescribing Characteristics
Postoperative APM exposure was defined using date-stamped billing codes for a conventional or “typical” APM (haloperidol) or newer or “atypical” APMs (olanzapine, quetiapine, risperidone, aripiprazole, and ziprasidone). These APMs were chosen based on clinical trials of delirium prevention and treatment, and common use in routine practice for this indication7,25,26; other APMs that are typically used as antiemetics were excluded. In our prior work,27 postoperative APM use has 99% specificity and 92% positive predictive value to identify delirium when validated against the Confusion Assessment Method28 in cardiac surgical patients. The treatment duration and total daily dose were extracted. Since no APM dosing guideline exists for hospitalized or delirious patients, we followed a previously used approach29 that defined potentially excessive dosing according to the dosing guidelines in the CMS long-term care manual for dementia patients: haloperidol >2 mg/day, olanzapine >5 mg/day, quetiapine >150 mg/day, risperidone >2mg/day, and aripiprazole >10 mg/day.30 Potentially excessive dosing for ziprasidone, which was not included in the CMS manual, was defined as >160 mg/day, the maximum maintenance dose.31 We also examined first APM exposure in the intensive care unit (ICU), exposure on the day of discharge, and treatment duration longer than 7 days.
Hospital APM Prescribing Intensity
The hospital APM prescribing intensity was defined as the proportion of patients who were newly treated with APMs in the postoperative period at each hospital. This was estimated from a mixed-effects logistic model that included the hospital identifier as a normally distributed random intercept. These predicted intercepts are empirical Bayes estimates that account for random variation.32 Hospitals were classified into quintiles (≤4.4%, 4.5–5.7%, 5.8–7.3%, 7.4–9.4%, or ≥9.5%). We also estimated the adjusted prescribing intensity from a mixed-effects model that included patient and hospital characteristics (listed below). This represents the variation across hospitals that is not explained by patient and hospital characteristics.
Patient and Hospital Characteristics
We examined the following variables that might affect a patient’s risk of APM exposure based on clinical knowledge and previous literature22,33,34: age, sex, race, insurance, admission type, type of surgery, dementia, and delirium. Previously validated coding algorithms were used to identify dementia (sensitivity 32%, specificity 100%, positive predictive value 96%, negative predictive value 98%)35 and delirium (sensitivity 20%, specificity 99%, positive predictive value 91%, negative predictive value 66%).27 The Charlson Comorbidity Index was calculated.36 We measured clinical outcomes that could be affected by APMs, such as the length (days) of the index hospitalization, cardioversion or cardiopulmonary resuscitation, and in-hospital mortality. We also characterized hospitals in terms of number of beds, teaching status, location (urban vs rural), and geographical area. The hospital ICU model was defined as the ICU type (cardiac ICU, cardiovascular ICU, surgical ICU, general/medical ICU) that was used by a majority of cardiac surgical patients at each hospital during the study period.
Statistical Analysis
We examined the longitudinal trends of off-label APM use by summarizing the APM prescribing rates and characteristics over the calendar year and periods (2004–2006, 2007–2009, 2010–2012, 2013–2014). A time trend was examined by including a linear term for the time period in the generalized estimating equation (GEE) logistic regression model to account for clustering of patients within hospitals. We also assessed whether the longitudinal trends were consistent across subgroups defined by age, sex, race, delirium diagnosis, or comorbidity burden. In addition, we examined the variation in the unadjusted and adjusted APM prescribing rates across 465 hospitals. Patient and hospital characteristics were compared across the hospitals in different APM-prescribing quintiles using Kruskal-Wallis test and chi-square test. We tested whether patients treated at higher prescribing hospitals had worse outcomes than those at lower prescribing hospitals using GEE poisson regression for the mean length of hospitalization and GEE logistic regression for cardioversion or cardiopulmonary resuscitation and in-hospital mortality. To identify predictors of exposure to any APM dose and potentially excessive dose, we used GEE logistic regression models that included the above-listed patient and hospital characteristics, except for outcome variables. Finally, we performed 2 sensitivity analyses. Since the longitudinal trends could have been affected by a change in hospitals included in the dataset over time, we examined the trends using data from 163 hospitals that contributed data for at least 3 of the 4 periods. We also repeated analysis without excluding 1,193 patients who received an APM on the day of surgery. Analyses were conducted using R software version 3.4.1. A 2-sided p-value <0.05 was considered statistically significant.
RESULTS
Longitudinal Trends in Postoperative APM Use
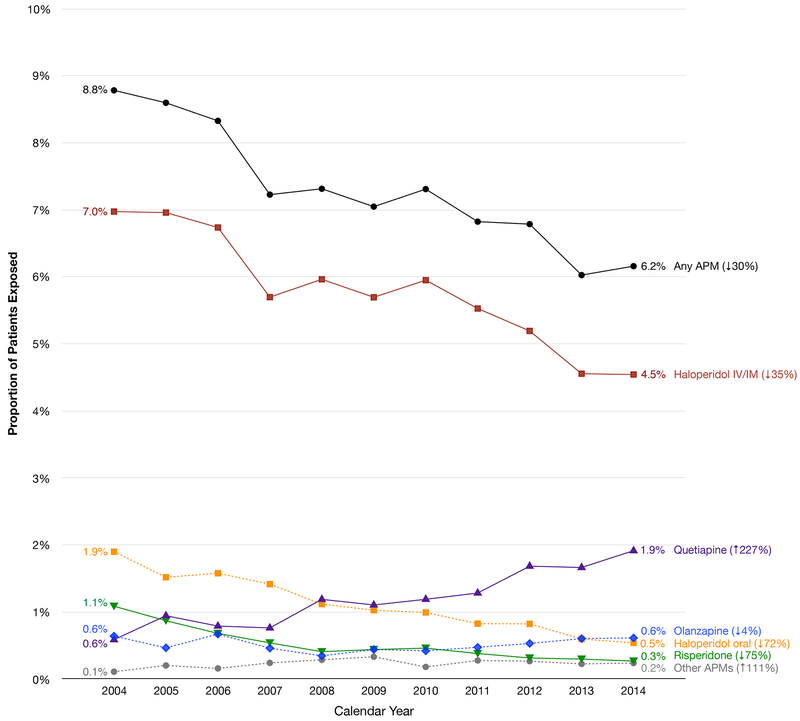
Our study population included 293,212 patients from 465 hospitals who underwent cardiac surgery and were not using APMs while in the hospital prior to or on the day of surgery (Supplementary Figure). The overall rate of APM use in this population was 7.3%. The rate declined from 8.8% in 2004 to 6.2% in 2014 (p<0.001), representing a 30% relative decrease (Figure 1). This trend was driven by reductions in use of haloperidol (parenteral: 7.0% to 4.5%, p<0.001; oral: 1.9% to 0.5%, p<0.001) and risperidone (1.1% to 0.3%, p<0.001). However, quetiapine use rose steeply (0.6% to 1.9%, p=0.03). This trend was consistent across the subgroups defined by age, sex, race, delirium diagnosis, or comorbidity burden, and in sensitivity analyses that only included hospitals contributing data for 3 or more calendar periods and that included patients who were treated on the day of surgery (data not shown).
Figure 1. Longitudinal Trends in Antipsychotic Medication Use After Cardiac Surgery, Premier Database 2004–2014.
The data from Premier Database 2004–2014 show a progressive decline in postoperative antipsychotic medication use in older cardiac surgical patients (p<0.001). Although use of haloperidol and risperidone has declined (p<0.001 for both), quetiapine use has tripled over time (p=0.03). The prescribing trends of olanzapine (p=0.44) and other antipsychotic medications (aripiprazole and ziprasidone) (p=0.47) did not change significantly.
Among APM-treated patients (Table 1), a large majority received haloperidol (85.6%). Common choice for atypical APMs shifted from risperidone (10.4%) in 2004–2006 to quetiapine (29.1%) in 2013–2014. There was a decline in the mean daily dose of haloperidol, quetiapine, and ziprasidone, while the mean daily dose of the other APMs remained essentially unchanged. The rate of potentially excessive dosing (per 100 person-days) was 56.0%, with the highest rate for haloperidol (91.1%). The rate declined from 60.7% to 44.9% (p<0.001), particularly for quetiapine (9.4% to 3.3%; p<0.001) and aripiprazole (52.5% to 13.3%; p<0.001). Most treated patients initiated APMs in the ICU, and one in eight treated patients received APMs on the day of discharge. The mean treatment duration was 4.6 days and 15.5% received APMs more than 7 days. These patterns were consistent throughout the study period.
Table 1.
Prescribing Characteristics of Antipsychotic Medications in Older Patients After Cardiac Surgery, Premier Database 2004–2014a
| Characteristics | Total | 2004 −2006 |
2007 −2009 |
2010 −2012 |
2013 −2014 |
P value |
|---|---|---|---|---|---|---|
| Number of hospitals | 465 | 205 | 369 | 255 | 237 | NA |
| Number of patients | 293,212 | 81,009 | 77,632 | 83,856 | 50,715 | NA |
| APM exposure, % | 7.3 | 8.6 | 7.2 | 7.0 | 6.1 | <0.001 |
| Excessive doseb, per 100 pd | 56.0 | 60.7 | 59.1 | 53.1 | 44.9 | <0.001 |
| Haloperidol, % | 85.6 | 88.5 | 87.6 | 84.3 | 78.2 | <0.001 |
| Mean daily dose, mg | 10.0 | 11.0 | 10.0 | 9.5 | 8.4 | <0.001 |
| Excessive doseb, per 100 pd | 91.1 | 90.5 | 90.0 | 92.5 | 92.4 | 0.24 |
| Olanzapine, % | 7.1 | 7.0 | 5.8 | 6.9 | 10.0 | 0.23 |
| Mean daily dose, mg | 8.2 | 7.5 | 8.8 | 7.9 | 8.8 | 0.30 |
| Excessive doseb, per 100 pd | 38.1 | 36.9 | 40.5 | 38.4 | 37.5 | 0.92 |
| Quetiapine, % | 16.4 | 9.1 | 14.3 | 20.1 | 29.1 | <0.001 |
| Mean daily dose, mg | 57.0 | 66.0 | 71.4 | 51.6 | 45.0 | 0.004 |
| Excessive doseb, per 100 pd | 8.1 | 9.4 | 15.3 | 5.6 | 3.3 | <0.001 |
| Risperidone, % | 7.2 | 10.4 | 6.5 | 5.5 | 4.7 | <0.001 |
| Mean daily dose, mg | 1.3 | 1.3 | 1.2 | 1.2 | 1.1 | 0.10 |
| Excessive doseb, per 100 pd | 8.1 | 9.0 | 5.4 | 9.1 | 8.1 | 0.85 |
| Aripiprazole, % | 0.6 | 0.3 | 0.7 | 0.9 | 0.9 | 0.002 |
| Mean daily dose, mg | 12.6 | 16.6 | 14.0 | 11.1 | 10.8 | 0.10 |
| Excessive doseb, per 100 pd | 30.3 | 52.5 | 44.3 | 16.8 | 13.3 | <0.001 |
| Ziprasidone, % | 2.5 | 1.5 | 3.4 | 2.6 | 2.9 | 0.10 |
| Mean daily dose, mg | 37.7 | 35.6 | 43.0 | 37.1 | 30.3 | 0.03 |
| Excessive doseb, per 100 pd | 0.6 | 0.0 | 1.0 | 1.0 | 0.0 | 0.40 |
| First dose given in ICU, % | 80.8 | 79.3 | 80.6 | 81.3 | 83.6 | 0.45 |
| Last dose on the discharge day, % | 12.5 | 11.1 | 14.0 | 13.5 | 10.9 | 0.66 |
| Duration, d, mean ± SD | 4.6 ± 7.2 | 4.8 ± 8.1 | 4.4 ± 7.1 | 4.5 ± 6.6 | 4.5 ± 6.2 | 0.29 |
| Prolonged use (duration >7 d), % | 15.5 | 16.2 | 14.7 | 15.2 | 16.1 | 0.83 |
Abbreviations: APM, antipsychotic medication; d, days; ICU, intensive care unit; IQR, interquartile range; NA, not applicable; pd, person-days; SD, standard deviation.
Except for the overall antipsychotic medication exposure, all the presented statistics were calculated among patients who were treated with antipsychotic medications.
Potentially excessive dose was defined according to the dosing guidelines in the Centers for Medicare and Medicaid Services long-term care facility manual for off-label use (haloperidol >2mg/day, olanzapine >5mg/day, quetiapine >150mg/day, risperidone >2mg/day, and aripiprazole >10mg/day) or the package insert (ziprasidone >160mg/day).
Hospital-Level Variation in Postoperative APM Use
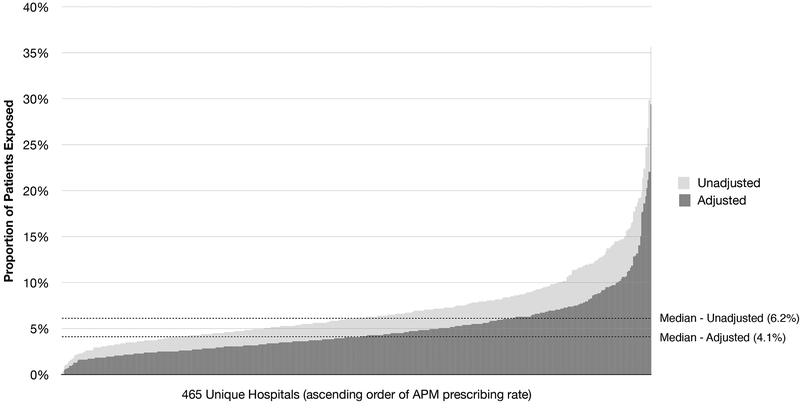
The hospital APM-prescribing intensity varied substantially from 0.3% to 35.6% across 465 hospitals (Figure 2). Adjustment for patient and hospital characteristics modestly reduced the variation (0.1% to 29.5%). There were noteworthy differences in prescribing characteristics among hospitals in different APM-prescribing quintiles (Supplementary Table). Higher prescribing hospitals used more haloperidol (highest vs lowest quintile: 87.4% vs 81.3%; p<0.001) and quetiapine (19.9% vs 12.8%; p<0.001). Patients treated at higher prescribing hospitals were less likely to initiate APMs in the ICU (78.0% vs 83.0%), but they were more likely to be treated on the day of discharge (15.1% vs 9.6%) and for a longer duration (4.8 vs 3.7 days) (p<0.001 for all comparisons). Patients were more likely to be of non-white race (22.6% vs 19.3%), have a commercial insurance (11.2% vs 7.6%), undergo non-elective (49.2% vs 46.2%) or valve/combined surgery (32.7% vs 28.9%), and have a delirium diagnosis (5.0% vs 4.1%) (p<0.001 for all comparisons). High prescribing hospitals tended to be teaching hospitals (52.7% vs 35.5%; p=0.049) and in the urban area (94.6% vs 86.0%; p=0.052). After adjusting for patient and hospital characteristics, patients at higher prescribing hospitals had a longer hospitalization (mean from quintile 1 to quintile 5: 11.7, 11.6, 11.7, 11.9, 12.3 days; p=0.004) and a greater risk of cardioversion or cardiopulmonary resuscitation (risk from quintile 1 to quintile 5: 3.0%, 3.2%, 2.3%, 2.9%, 3.5%; p=0.004); but in-hospital mortality was not significantly different (3.8%, 3.6%, 3.9%, 3.9%, 4.1%; p=0.20).
Figure 2. Hospital Variation in Antipsychotic Medication Use After Cardiac Surgery, Premier Database 2004–2014.
Abbreviation: APM, antipsychotic medication. The light grey bars indicate the unadjusted hospital-level antipsychotic prescribing rate (median, 6.2%; interquartile range, 4.5–8.4%) in the ascending order. The dark grey bars indicate the adjusted antipsychotic prescribing rate (median, 4.1%; interquartile range, 2.9–6.1%) which corresponds to the risk of antipsychotic exposure for a 73-year-old white man with Medicare insurance who is undergoing an elective coronary bypass grafting surgery at a small, rural, non-teaching hospital in the Northeast region.
Patient and Hospital Characteristics Associated with APM Exposure
Patient characteristics that were positively associated with APM exposure were age ≥75 years, Medicaid (vs Medicare), urgent/emergent surgery, valve/combined surgery, dementia, delirium, and high comorbidity burden (Table 2). Of these, delirium was the strongest risk factor for APM exposure (odds ratio, 9.73; 95% confidence interval, 9.02 to 10.5). Female sex and commercial insurance were associated with less APM exposure. None of the hospital characteristics were significantly associated with APM use. Similar patient characteristics were associated with potentially excessive dosing. Among the hospital characteristics, patients treated at hospitals whose ICU model was cardiovascular ICU had a lower risk for potentially excessive dosing.
Table 2.
Patient and Hospital Characteristics Associated with Exposure to Any Dose or Potentially Excessive Dose of Antipsychotic Medications After Cardiac Surgery, Premier Database 2004–2014
| Characteristics | N | Any Dose | Potentially Excessive Dosea | ||||
|---|---|---|---|---|---|---|---|
| Risk (%) | ORb | 95% CI | Risk (%) | ORb | 95% CI | ||
| Age | |||||||
| < 75 years | 163,026 | 5.9 | 1.00 | Reference | 4.9 | 1.00 | Reference |
| ≥ 75 years | 130,186 | 9.1 | 1.39 | (1.34, 1.44) | 7.7 | 1.40 | (1.35, 1.46) |
| Sex | |||||||
| Male | 191,436 | 7.8 | 1.00 | Reference | 6.6 | 1.00 | Reference |
| Female | 101,776 | 6.5 | 0.79 | (0.77, 0.82) | 5.3 | 0.77 | (0.74, 0.79) |
| Race | |||||||
| White | 221,646 | 7.2 | 1.00 | Reference | 6.0 | 1.00 | Reference |
| African American | 14,310 | 7.3 | 0.96 | (0.89, 1.04) | 6.4 | 1.00 | (0.92, 1.09) |
| Others | 57,256 | 7.8 | 0.97 | (0.92, 1.02) | 6.6 | 0.98 | (0.93, 1.04) |
| Primary insurance | |||||||
| Medicare | 258,989 | 7.4 | 1.00 | Reference | 6.2 | 1.00 | Reference |
| Medicaid | 3,677 | 8.6 | 1.23 | (1.09, 1.39) | 7.4 | 1.26 | (1.09, 1.45) |
| Commercial | 26,379 | 6.3 | 0.90 | (0.85, 0.94) | 5.4 | 0.91 | (0.86, 0.96) |
| Self-pay or other | 4,167 | 7.0 | 1.05 | (0.94, 1.16) | 5.7 | 0.98 | (0.87, 1.11) |
| Admission type | |||||||
| Elective | 156,223 | 6.8 | 1.00 | Reference | 5.8 | 1.00 | Reference |
| Urgent | 65,237 | 7.5 | 1.06 | (1.01, 1.11) | 6.3 | 1.03 | (0.99, 1.08) |
| Emergent | 70,266 | 8.2 | 1.12 | (1.08, 1.16) | 6.9 | 1.09 | (1.05, 1.13) |
| Other | 1,486 | 8.1 | 1.14 | (0.85, 1.51) | 5.5 | 0.87 | (0.69, 1.09) |
| Type of surgery | |||||||
| CABG | 202,572 | 6.6 | 1.00 | Reference | 5.6 | 1.00 | Reference |
| Valve surgery | 49,569 | 7.9 | 1.24 | (1.19, 1.29) | 6.6 | 1.22 | (1.16, 1.27) |
| Combined surgery | 41,071 | 10.2 | 1.45 | (1.39, 1.51) | 8.6 | 1.43 | (1.37, 1.49) |
| Diagnosis of dementia | |||||||
| Absent | 291,802 | 7.2 | 1.00 | Reference | 6.1 | 1.00 | Reference |
| Present | 1,410 | 27.3 | 3.42 | (3.01, 3.88) | 22.1 | 3.01 | (2.61, 3.47) |
| Diagnosis of delirium | |||||||
| Absent | 280,353 | 5.8 | 1.00 | Reference | 4.9 | 1.00 | Reference |
| Present | 12,859 | 40.1 | 9.73 | (9.02, 10.5) | 34.5 | 9.33 | (8.66, 10.0) |
| CCI | |||||||
| < 3 | 183,401 | 6.2 | 1.00 | Reference | 5.2 | 1.00 | Reference |
| ≥ 3 | 109,811 | 9.3 | 1.44 | (1.38, 1.49) | 7.7 | 1.41 | (1.36, 1.47) |
| Hospital bed size | |||||||
| < 393 beds | 99,950 | 6.9 | 1.00 | Reference | 5.7 | 1.00 | Reference |
| ≥ 393 beds | 193,262 | 7.5 | 0.94 | (0.80–1.10) | 6.4 | 0.97 | (0.83–1.14) |
| Teaching status | |||||||
| Non-teaching | 126,514 | 6.8 | 1.00 | Reference | 5.7 | 1.00 | Reference |
| Teaching | 166,698 | 7.7 | 1.13 | (0.96, 1.34) | 6.5 | 1.09 | (0.92, 1.28) |
| Location | |||||||
| Rural | 20,551 | 5.6 | 1.00 | Reference | 4.8 | 1.00 | Reference |
| Urban | 272,661 | 7.4 | 1.11 | (0.89, 1.39) | 6.3 | 1.11 | (0.88, 1.40) |
| Hospital ICU model | |||||||
| CICU | 81,636 | 7.7 | 1.00 | Reference | 6.6 | 1.00 | Reference |
| CVICU | 64,315 | 6.3 | 0.72 | (0.51, 1.01) | 5.2 | 0.68 | (0.49, 0.93) |
| ICU/MICU | 118,115 | 7.4 | 0.90 | (0.74, 1.09) | 6.1 | 0.86 | (0.72, 1.04) |
| SICU | 29,146 | 8.4 | 1.00 | (0.74, 1.36) | 7.2 | 1.00 | (0.73, 1.36) |
Abbreviations: APM, antipsychotic medication; CABG, coronary artery bypass grafting; CICU, cardiac intensive care unit; CCI, Charlson Comorbidity Index; CI, confidence interval; CVICU, cardiovascular intensive care unit; ICU, intensive care unit; MICU, medical intensive care unit; OR, odds ratio; SICU, surgical intensive care unit.
Potentially excessive dose was defined according to the dosing guidelines in the Centers for Medicare and Medicaid Services long-term care facility manual for off-label use (haloperidol >2mg/day, olanzapine >5mg/day, quetiapine >150mg/day, risperidone >2mg/day, and aripiprazole >10mg/day) or the package insert (ziprasidone >160mg/day).
Odds ratios and 95% confidence intervals were adjusted for all the variables listed in the table, calendar years, and geographic areas using generalized estimating equation logistic regression.
DISCUSSION
To our knowledge, this is the first study to examine the temporal trends and variation of off-label APM use in a nationwide sample of older cardiac surgical patients over 11 years. Despite a downward trend, 6.2% of patients were treated with APMs after cardiac surgery in 2014, which corresponds to almost 10,000 patients.37 Although haloperidol remained the most commonly prescribed APM, we observed a shift in choice of atypical APMs from risperidone to quetiapine. The steep increase in quetiapine use and consistently high rate of potentially excessive dosing of haloperidol are worrisome, particularly in light of recent guidelines which highlighted the lack of consistent evidence on the benefit of APMs for delirium4–6 as well as their potential harm.7,38 One in eight treated patients received APMs on the day of discharge, which may indicate continued exposure after discharge. Moreover, the wide unexplained variation of APM use across hospitals suggests different prescribing cultures and raises concerns for inappropriate use.33 Collectively, our results underscore a need to promote more judicious APM use in the postoperative period after cardiac surgery.
Previous research on off-label APM prescribing has been mainly conducted in older adults with dementia and nursing home residents. A recent national survey of US nursing homes reported a decreasing trend in off-label APM use from 24% in 2011 to 16% in 2016.22 The facility-level prescribing rates in 2005 ranged from ≤24% in the lowest prescribing quintile to ≥44% in the highest quintile.34 An earlier 2003 study of nursing homes in Ontario, Canada, showed a similar variation in APM use from 21% in the lowest quintile to 44% in the highest quintile.33 Most residents received atypical APMs (quetiapine, risperidone, olanzapine).32,33,39 The increased risk of mortality and other serious adverse events has been documented for both typical and atypical APMs.9–19
The utilization of APMs in hospitalized patients without psychiatric illnesses has been investigated in only a few studies. Two single-center studies found a similar 9% rate of off-label APM use during non-psychiatric hospitalization.40,41 Atypical APMs were more commonly prescribed than haloperidol, and they were more likely to be continued at discharge.40 In another US study that analyzed over 2.6 million non-psychiatric hospitalizations, APMs were prescribed to 6% of medical and surgical patients.29 Although the rate of APM use was higher in medical patients, due to a higher volume of surgical admissions, a larger absolute number of surgical patients received APMs than did medical patients. The prescribing rate ranged from 3% in the lowest prescribing quintile to 9% in the highest quintile. As in the present study, this variation was not fully explained by patient characteristics treated at individual hospitals.29
The risk of adverse events associated with APMs in hospitalized surgical patients may differ from the risk associated with APM use in dementia patients, because treatment duration is usually shorter when APMs are used to treat delirium. Hospitalized cardiac surgical patients may have different vulnerability to adverse events from patients with dementia. Nonetheless, available safety data in hospitalized patients are limited. In a recent study of 3,706 patients who were treated off-label with APMs after cardiac surgery, both typical and atypical APMs were equally harmful in terms of mortality, cardiac arrhythmia, and pneumonia; moreover, the risk of adverse neurologic events was higher for atypical APMs, particularly quetiapine, relative to haloperidol.42 Clinical trials had limited statistical power to examine adverse events.7,8
Our study raises several concerns about the recent prescribing trends of APMs in the postoperative period after cardiac surgery. Although the observed downtrend coincides with publication of key safety studies9–19 and the Food and Drug Administration Boxed Warnings to atypical APMs in 200543 and typical APMs in 200844 in older people with dementia, quetiapine use tripled. Quetiapine has anti-histamine and anti-serotonergic properties which can cause sedation. We speculate that low-dose quetiapine may be increasingly used for insomnia45—a highly prevalent condition in hospitalized patients46,47 and an important risk factor for delirium48—despite its unclear efficacy and potential harms.49 Moreover, potentially excessive dosing was prevalent, particularly for haloperidol. While the CMS long-term care dosing guideline30 may not be appropriate for hospitalized patients, the harmful effects of APMs generally increase with greater dose.10,11 The variation in APM use in our study (highest vs lowest quintile: 14% vs 3%) was greater than the previously reported variation in the overall hospitalized population (9% vs 3%).29 This may represent more variability in the clinical approach to prescribing APMs among providers in cardiac surgical services than in non-surgical services. Patients treated at higher prescribing hospitals were more likely to initiate APMs in the non-ICU setting and to be treated for a longer duration and at discharge. Longer duration of hospitalization and higher rate of cardioversion or cardiopulmonary resuscitation were also worrisome, although these small differences might have been due to incomplete adjustment for patient characteristics. Finally, we found that delirium was the strongest risk factor for APM use, which suggests that delirium prevention is crucial to reduce off-label APM exposure.
There are several caveats to consider in interpreting our study. First, the Premier Database does not contain information about outpatient medication use or indications for APM use. Some use might have been clinically appropriate. In addition, off-label use or doses higher than the CMS long-term care guideline may be justified for management of severe symptoms of delirium that can cause harm or interrupt life-sustaining treatments. Second, the dose of APMs recorded in the database may not be the actual dose administered to patients. If a partial dose (e.g., less than a full vial of intravenous haloperidol) had been administered, the daily dose could have been overestimated. Moreover, we were unable to distinguish a scheduled dose from an as-needed dose. Third, diagnoses recorded in the Premier Database may not have been accurate or complete. For instance, hyperactive delirium is more likely to be recorded than hypoactive delirium.27 Diagnoses from the index hospitalization may not have adequately captured relevant chronic conditions for accurate estimation of CCI. As a result, some hospital-level variation might have been due to different patient characteristics or hospital practice (e.g., systematic delirium screening) that were not measured. Finally, it is unclear whether our findings can be extended to patients undergoing other major surgeries.
In hospitalized older patients after cardiac surgery, we found that the rates of off-label APM use and potentially excessive dosing has declined, but substantial hospital-level variation and rapidly increasing trend in quetiapine use are concerning. Further research is needed to examine whether the reduced use of APMs resulted in more use of benzodiazepines or hypnotics. Continued use of APMs in the post-acute settings warrants additional research. Since our findings predate the recent guidelines published by the Society of Critical Care Medicine5 and the American Geriatrics Society Expert Panel,6 it will be useful to examine future prescribing trends to assess their impact. To promote appropriate APM prescribing and improve clinical outcomes of older cardiac surgical patients, high-quality evidence on the effectiveness and harm of APMs for management of delirium and training of health care providers about effective non-pharmacological interventions50 are urgently needed.
Supplementary Material
Supplementary Table. Patient and Hospital Characteristics According to Hospital Prescribing Intensity, Premier Database 2004–2014
Supplementary Figure. Selection of Study Population
ACKNOWLEDGMENT
Conflict of Interest and Funding Support:
Dr. Kim is supported by Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies. He is also supported by the Boston Claude D. Pepper Older Americans Independence Center/Pilot and Exploratory Studies Core (P30AG031679) and Boston Roybal Center Pilot Award (P30AG048785). He is a consultant to Alosa Health, a nonprofit educational organization with no relationship to any drug or device manufacturers.
Dr. Bateman is supported by grant K08HD075831 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. Dr. Bateman is an investigator on grants to the Brigham and Women’s Hospital from Pfizer, Lilly, GlaxoSmithKline, and Baxalta for projects unrelated to the present study, and is a consultant to Optum, Inc.
Dr. Huybrechts is supported by grant K01MH099141 from the National Institute of Mental Health. Dr. Huybrechts is an investigator on grants to the Brigham and Women’s Hospital from Pfizer, Lilly, and GlaxoSmithKline for projects unrelated to the present study.
Dr. Inouye is supported by grants P01AG031720, R24AG054259, R01AG044518, and K07AG041835 from the National Institute on Aging.
Dr. Marcantonio is funded by grants R01AG030618, R01AG051658, and a Mid-Career Investigator Award (K24AG035075) from the National Institute on Aging.
Dr. Herzig is funded by grant K23AG042459 from the National Institute on Aging.
Dr. Ely is supported by the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC, Nashville, TN) and by grants R01AG027472, R01AG035117, R01HL111111, and R01GM120484 from the National Institutes of Health. Dr. Ely has conducted CME activities sponsored by Abbott, Hospira, and Orion.
Other coauthors have no conflicts to disclose.
Sponsor’s Role: The funding sources did not have any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Meeting presentations: An earlier version of this work was presented as an abstract at the 7th Annual American Delirium Society Conference in Nashville, TN, USA, on June 5, 2017, and at the 33rd International Conference on Pharmacoepidemiology & Therapeutic Risk Management in Montreal, QC, Canada, on August 29, 2017.
Impact Statement: We certify that this work is novel. To our knowledge, the longitudinal trends and hospital variation in postoperative antipsychotic use have not been described. The inpatient use of off-label antipsychotics is being considered as a hospital quality measure by CMS. Our study provides timely information on highly variable prescribing cultures at hospitals and raise concerns about inappropriate prescribing.
REFERENCES
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med. 2017;377(15):1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. Jama. 2012;308(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Mahony R, Murthy L, Akunne A, Young J, Guideline Development G. Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Ann Intern Med. 2011;154(11):746–751. [DOI] [PubMed] [Google Scholar]
- 5.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. [DOI] [PubMed] [Google Scholar]
- 6.American Geriatrics Society Expert Panel on Postoperative Delirium in Older A. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic Medication for Prevention and Treatment of Delirium in Hospitalized Adults: A Systematic Review and Meta-Analysis. J Am Geriatr Soc. 2016;64(4):705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775–786. [DOI] [PubMed] [Google Scholar]
- 10.Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. Bmj. 2012;344:e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335–2341. [DOI] [PubMed] [Google Scholar]
- 13.Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang PS. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Cmaj. 2007;176(5):627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerhard T, Huybrechts K, Olfson M, et al. Comparative mortality risks of antipsychotic medications in community-dwelling older adults. Br J Psychiatry. 2014;205(1):44–51. [DOI] [PubMed] [Google Scholar]
- 15.Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setoguchi S, Wang PS, Alan Brookhart M, Canning CF, Kaci L, Schneeweiss S. Potential causes of higher mortality in elderly users of conventional and atypical antipsychotic medications. J Am Geriatr Soc. 2008;56(9):1644–1650. [DOI] [PubMed] [Google Scholar]
- 17.Rochon PA, Normand SL, Gomes T, et al. Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med. 2008;168(10):1090–1096. [DOI] [PubMed] [Google Scholar]
- 18.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. Jama. 2005;294(15):1934–1943. [DOI] [PubMed] [Google Scholar]
- 19.Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38(5):553–563. [DOI] [PubMed] [Google Scholar]
- 20.Roach GW, Kanchuger M, Mangano CM, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335(25):1857–1863. [DOI] [PubMed] [Google Scholar]
- 21.Hammermeister KE, Burchfiel C, Johnson R, Grover FL. Identification of patients at greatest risk for developing major complications at cardiac surgery. Circulation. 1990;82(5 Suppl):IV380–389. [PubMed] [Google Scholar]
- 22.CMS Measure. National Partnership to Improve Dementia Care in Nursing Homes: Antipsychotic Medication Use Data Report (July 2016). 2016; https://nhqualitycampaign.org/files/AP_package_20160715.pdf, Aug 15, 2016.
- 23.Use of Antipsychotics in Older Patients in the Inpatient Hospital Setting (AP) Measure. 2016; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/Hospital-MDM_AP-Public-Comment-Summary-Report_FINAL.pdf, September 25, 2016.
- 24.Premier, Inc. Available at https://learn.premierinc.com/i/790965-premier-healthcare-database-whitepaper/0?. Accessed January 9, 2018.
- 25.Markowitz JD, Narasimhan M. Delirium and antipsychotics: a systematic review of epidemiology and somatic treatment options. Psychiatry (Edgmont). 2008;5(10):29–36. [PMC free article] [PubMed] [Google Scholar]
- 26.Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DH, Lee J, Kim CA, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf. 2017;26:945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 29.Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med. 2016;11(8):543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.State Operations Manual Appendix PP - Guidance to Surveyors for Long Term Care Facilities. Available at: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_pp_guidelines_ltcf.pdf. Accessed February 21, 2017.
- 31.Geodon. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020825s035,020919s023lbl.pdf. Accessed February 21, 2017.
- 32.Huybrechts KF, Rothman KJ, Brookhart MA, et al. Variation in antipsychotic treatment choice across US nursing homes. J Clin Psychopharmacol. 2012;32(1):11–17. [DOI] [PubMed] [Google Scholar]
- 33.Rochon PA, Stukel TA, Bronskill SE, et al. Variation in nursing home antipsychotic prescribing rates. Arch Intern Med. 2007;167(7):676–683. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Briesacher BA, Field TS, Tjia J, Lau DT, Gurwitz JH. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J.Clin.Epidemiol 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 37.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: 2016. Accessed March 13, 2017 https://www.cdc.gov/nchs/data/hus/hus15.pdf#096. [PubMed] [Google Scholar]
- 38.Agar MR, Lawlor PG, Quinn S, et al. Efficacy of Oral Risperidone, Haloperidol, or Placebo for Symptoms of Delirium Among Patients in Palliative Care: A Randomized Clinical Trial. JAMA Intern Med. 2017;177(1):34–42. [DOI] [PubMed] [Google Scholar]
- 39.Briesacher BA, Tjia J, Field T, Peterson D, Gurwitz JH. Antipsychotic use among nursing home residents. Jama. 2013;309(5):440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herzig SJ, Rothberg MB, Guess JR, et al. Antipsychotic Use in Hospitalized Adults: Rates, Indications, and Predictors. J Am Geriatr Soc. 2016;64(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loh KP, Ramdass S, Garb JL, Brennan MJ, Lindenauer PK, Lagu T. From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim DH, Huybrechts KF, Patorno E, et al. Adverse Events Associated with Antipsychotic Use in Hospitalized Older Adults After Cardiac Surgery. J Am Geriatr Soc. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Information for Healthcare Professionals: Conventional Antipsychotics. https://wayback.archive-it.org/7993/20170404172011/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107211.htm, April 16, 2016.
- 44.Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. https://wayback.archive-it.org/7993/20170723113222/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm, April 16, 2016.
- 45.Desforges P, Lee TC, McDonald EG. Insomnia in the Hospital-Not Just a Bad Dream. JAMA Intern Med. 2016;176(9):1253. [DOI] [PubMed] [Google Scholar]
- 46.Isaia G, Corsinovi L, Bo M, et al. Insomnia among hospitalized elderly patients: prevalence, clinical characteristics and risk factors. Arch Gerontol Geriatr. 2011;52(2):133–137. [DOI] [PubMed] [Google Scholar]
- 47.Young JS, Bourgeois JA, Hilty DM, Hardin KA. Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008;3(6):473–482. [DOI] [PubMed] [Google Scholar]
- 48.Todd OM, Gelrich L, MacLullich AM, Driessen M, Thomas C, Kreisel SH. Sleep Disruption at Home As an Independent Risk Factor for Postoperative Delirium. J Am Geriatr Soc. 2017. [DOI] [PubMed] [Google Scholar]
- 49.Anderson SL, Vande Griend JP. Quetiapine for insomnia: A review of the literature. Am J Health Syst Pharm. 2014;71(5):394–402. [DOI] [PubMed] [Google Scholar]
- 50.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table. Patient and Hospital Characteristics According to Hospital Prescribing Intensity, Premier Database 2004–2014
Supplementary Figure. Selection of Study Population