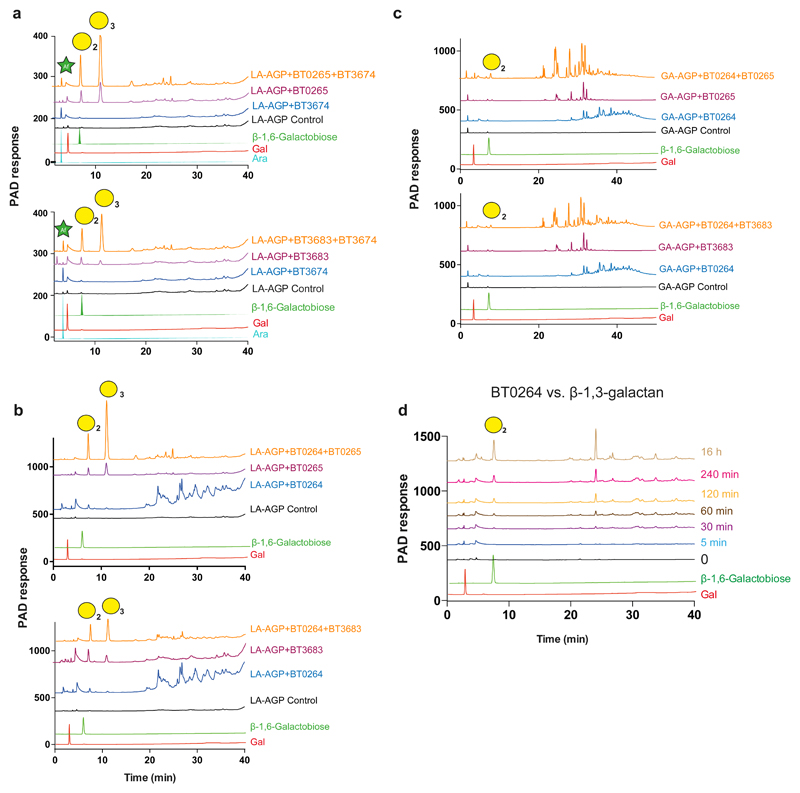
Figure 2. HPAEC analysis of the activity of GH43_24 β1,3-D-galactanases.
The AGPs were at 5 mg/ml for all reactions except BT0264 against LA-AGP and BT3683 against GA-AGP, when substrate concentration was increased to 25 mg/ml, the β-1,3-galactan backbone was at 1.5 mg/ml. Enzyme concentration was 1 µM. Reactions were incubated for 16 h in 20 mM sodium phosphate buffer pH 7.0 containing 150 mM NaCl buffer. The data shown are representative of three independent replicates. a, reveals how the GH127 β-L-arabinofuranosidase BT3674 acts in synergy with the exo-β1,3-galactosidases BT0265 and BT3683 on LA-AGP. The synergy between the endo-β1,3-galactanase with BT0265 and BT3683 acting on LA-AGP and GA-AGP was shown in b and c, respectively. d, shows a time course of BT0264 acting on β-1,3-galactan. Peaks containing a defined galactooligosaccharide are identified by a yellow circle with the degree of polymerization shown in subscript. In b and c the peaks corresponding to β1,6-galactobiose and β1,6-galactotriose were identified by LC-MS (see Supplementary Fig. 1d), and the β1,6 linkage was revealed by sensitivity to the β1,6-galactosidase BT0290.