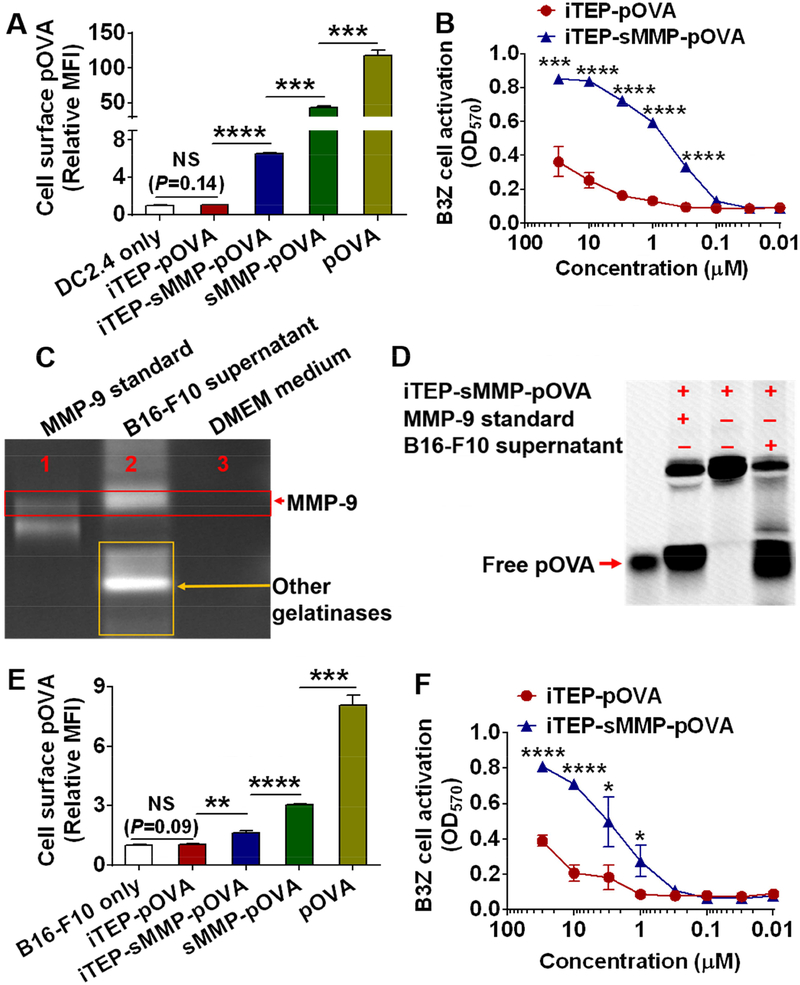
Figure 2.
iTEP-sMMP-pOVA resulted in greater pOVA presentation on the cell surface than iTEP-pOVA in vitro. (A) Relative MFI of DC2.4 cells. The cells were first incubated with pOVA, sMMP-pOVA, iTEP-sMMP-pOVA, iTEP-pOVA, or cell culture medium. The cells were then stained with an antibody specific to pOVA/MHC class I complexes on the cell surface. The relative MFI was generated by normalizing observed MFI of each sample against the MFI of DC2.4 cells that were incubated with cell culture medium. (B) An OD570 plot of B3Z cell indicated that iTEP-sMMP-pOVA generated more pOVA epitopes on DC2.4 surface than iTEP-pOVA. (C) A gel photo of the gelatin zymography assay showed that the culture supernatant of B16-F10 cells contained MMP-9. The top bright band indicated where MMP-9 migrated and cleaved gelatin. The additional bright bands in Lane 2 implied that the culture supernatant of B16-F10 cells contained other gelatinases. (D) A photo of a SDS-PAGE gel showed that a small peptide can be cleaved from iTEP-sMMP-pOVA in the presence of B16-F10 cell culture supernatant. When B16-F10 cells were used in the assay, iTEP-sMMP-pOVA still generated more pOVA epitopes onto the cell surface than iTEP-pOVA based on the flow cytometry assay (E) and B3Z cell activation assay (F). Experiments were conducted in triplicate. Data were shown as mean ± SD and analyzed by Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS = not significant.