Figure 2. Mitochondrial metabolism in immune cell polarization and innate immune responses.
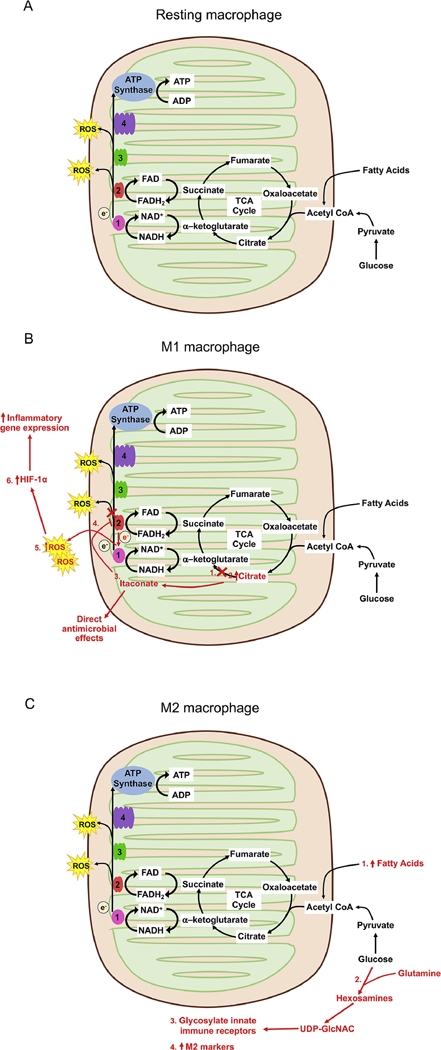
A. In resting macrophages, glucose and fatty acids are broken down to acetyl CoA that enters the TCA cycle. As the TCA cycle progresses, NADH and FADH2 are regenerated as electron donors for the ETC. Most electrons in the ETC are used to generate ATP although some leak off and combine with oxygen to create ROS. B. Activated M1 macrophages have modifications in their metabolism that drives their pro-inflammatory characteristics. (1) M1 macrophages downregulate isocitrate dehydrogenase, resulting in a block in the cycle moving forward from citrate. (2) Citrate accumulates. (3) The metabolic products of citrate are converted to itaconate by IRG1 (immune response gene 1 protein), which is markedly upregulated in M1 macrophages (4) In addition to direct antimicrobial effects, itaconate also inhibits complex II (also known as succinate dehydrogenase), preventing forward progression of the ETC. (5) Reverse transfer of electrons to complex 1 results in increased ROS generation. (6) increased ROS results in stabilization of the transcription factor HIF-1a with subsequent upregulation of inflammatory gene expression, including the potent inflammatory cytokine IL-1 β. C. The metabolic signature of activated M2 macrophages is necessary for their function. (1) M2 macrophages have enhanced fatty acid oxidation driving the TCA cycle to generate ATP. (2) M2 macrophages also require glycolysis, hydrolyzing glucose and using glutamine through the hexosamine pathway to generate uridine diphosphate (UDP)-N-acetylglucosamine (UDP- GIcNAc). This production of UDP-GIcNAc is necessary for (3) glycosolation of immune receptors and (4) the upregulation of expression of specific M2 markers.
