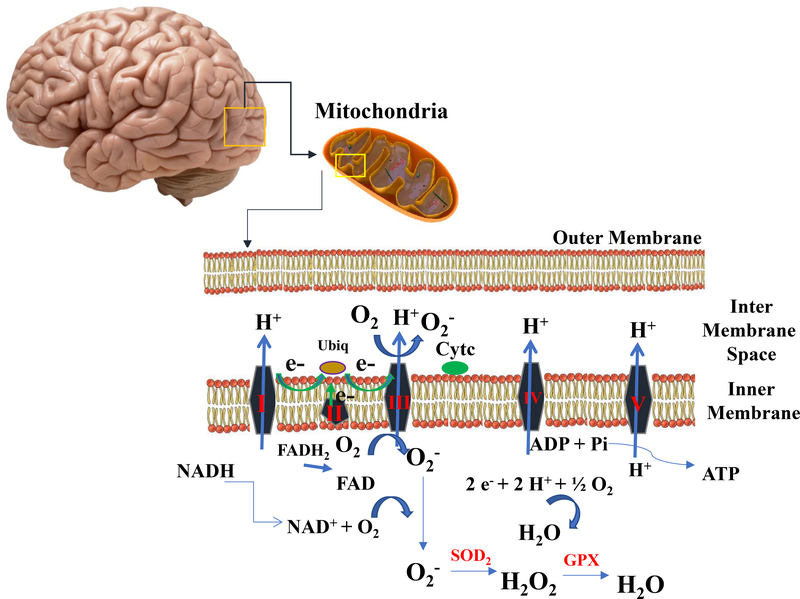
Figure 3. Formation and Neutralization of Reactive Oxygen Species in the Mitochondrial Electron Transport Chain (ETS).
The Krebs cycle is a series of enzymatic reactions that provide electrons (from pyruvate via acetyl CoA) to the ETS in the form of NADH and FADH2. These electrons then undergo vectorial transport along the ETS, generating an electrochemical energy gradient by which ADP can be phosphorylated to ATP at complex V. In order to maintain electron flow (and ATP generation) electrons must ultimately be ‘removed’ from the ETS, and this is accomplished at Complex IV (cytochrome oxidase) where the electrons reduce oxygen to water in four consecutive (but concerted) one-electron steps. Although the whole process is really rather efficient, some 1–2% of the molecular oxygen consumed during normal physiological respiration is reduced in one-electron side reactions (mostly at Complex ‘s I and III) into the superoxide anion radical, O2•- (also commonly just called ‘superoxide’). The O2•- so generated is almost immediately dismutated to hydrogen peroxide (H2O2) by superoxide dismutase (SOD) and, H2O2 can then be removed by the enzyme, glutathione peroxidase (GPX).