Abstract
Background
We previously showed that the presence of a CKD-associated locus in SHROOM3 in a donor kidney results in increased expression of SHROOM3 (an F-actin–binding protein important for epithelial morphogenesis, via rho-kinase [ROCK] binding); this facilitates TGF-b signaling and allograft fibrosis. However, other evidence suggests Shroom3 may have a protective role in glomerular development.
Methods
We used human data, Shroom3 knockdown podocytes, and inducible shRNA-mediated knockdown mice to study the role of Shroom3 in adult glomeruli.
Results
Expression data from the Nephroseq database showed glomerular and nonglomerular SHROOM3 had opposing associations with renal function in CKD biopsy samples. In human allografts, homozygosity at rs17319721, the SHROOM3 locus linked with lower GFR, was associated with reduced albuminuria by 2 years after transplant. Although our previous data showed reduced renal fibrosis with tubular Shroom3 knockdown, this study found that glomerular but not tubular Shroom3 knockdown induced albuminuria. Electron microscopy revealed diffuse foot process effacement, and glomerular RNA-sequencing showed enrichment of tyrosine kinase signaling and podocyte actin cytoskeleton pathways in knockdown mice. Screening SHROOM3-interacting proteins identified FYN (a src-kinase) as a candidate.We confirmed the interaction of endogenous SHROOM3 with FYN in human podocytes via a critical Src homology 3–binding domain, distinct from its ROCK-binding domain. Shroom3-Fyn interaction was required in vitro and in vivo for activation of Fyn kinase and downstream nephrin phosphorylation in podocytes. SHROOM3 knockdown altered podocyte morphology, cytoskeleton, adhesion, and migration.
Conclusions
We demonstrate a novel mechanism that may explain SHROOM3’s dichotomous associations in glomerular versus nonglomerular compartments in CKD
Keywords: Cell Signaling, podocyte, renal transplantation, gene expression
Visual Abstract
CKD is defined by reduced eGFR or proteinuria, and characterized by interstitial fibrosis and glomerulosclerosis, reflecting damage to different renal compartments. Genome-wide association studies (GWAS) have identified candidate susceptibility loci for CKD.1,2 Although mechanistic studies have ascribed functional characteristics to some of these loci,3 the detailed basis of these SNP-variant associations with renal function or histology remain largely undescribed. This hinders translation of GWAS information for the development of novel therapies.
We previously identified the TCF7L2-dependent enhancer function of the A-allele at rs17319721 in a cohort of renal allograft recipients from the genomics of allograft rejection study (GoCAR).4 Rs17319721, in the first intron Shroom3, is associated with CKD in GWAS.1,2,5 Shroom3 is an F-actin binding protein, important for epithelial morphogenesis via rho-kinase (ROCK) binding.6,7 We observed enhanced SHROOM3 expression in A-allele donors at 3-month biopsy, associated with increased interstitial fibrosis score (CADI score) in allografts by 1-year post transplant. Tubular-specific Shroom3 knockdown in mice inhibited renal fibrosis in a ureteric obstruction model. Addition of a ROCK inhibitor abrogated the enhanced collagen production when SHROOM3 was overexpressed in tubular cells, suggesting that the ROCK binding function of the ASD2-domain of SHROOM3 contributed to profibrotic effects. Recent work has confirmed the TCF7L2-dependent transcription of a Shroom3 isoform in the presence of the A-allele and identified a 14–3-3 binding site, which could facilitate profibrotic Hippo signaling in renal cells.8 These data suggested potential for testing the role of Shroom3 antagonism in renal tubules as a therapeutic strategy for renal fibrosis in A/A or A/G genotype allografts.
However, other data suggest a protective role for Shroom3 in glomerular development.9,10 In the fawn-hooded hypertensive rat model (FHH) of FSGS, mutations were identified within the Shroom3 locus, and the FSGS phenotype was reversed with wild-type Shroom3 gene.9 Homozygous Shroom3-knockout mice showed abnormal glomerulogenesis (e13.5–e18.5) and deficient myosin-II phosphorylation due to deficient ROCK-binding function of Shroom3.10 When human CKD-GWAS SNPs were examined for associations with albuminuria, the A-allele was surprisingly associated with a mild beneficial effect on albuminuria despite the increased risk of CKD.11
These data suggest a protective role for Shroom3 in proteinuria and glomerular development, but do not explain the association of the A-allele with CKD, or allograft nephropathy. We hypothesized that SHROOM3 may have dichotomous roles in glomerular and tubular compartments in order to explain the associations of the enhancer SNP with lower albuminuria, and lower GFR. We used inducible, tissue-specific, shRNA-mediated knockdown mice to examine the effect of Shroom3 antagonism in postdevelopmental glomeruli, to describe the phenotype and ascribe mechanism.
Methods
See Supplemental Material for details.
GoCAR Study
Details of the observational GoCAR cohort, including eligibility and exclusion criteria are published elsewhere.4 eGFR was calculated using Modified Diet in Renal Disease equation.12
SHROOM3 SNP Analysis
Targeted SNP genotyping for rs17319721 was done using TaqMan SNP analysis assay (#4351379; Applied Biosystems, Foster City, CA). DNA was extracted from preimplantation biopsies or blood for donor SNP and from peripheral blood for recipient SNP assay (approximately 20 ng per sample).
In Vitro Studies
Cells
Human podocytes (Dr. Moin Saleem), and HEK-293 cells were expanded using RPMI-1640 (1% ITS) and DMEM (Gibco) media.
Overexpression Studies
A human SHROOM3 construct, and shRNA lentivirus has been described.4 Site-specific mutagenesis replacing -CCC/-CCA (Proline) with -GCC/-GCA (Alanine) by proprietary recombinase (GenScript Inc., Piscataway, NJ). Cell adhesion assay was performed in β-Laminin–coated 96-well plates, as described.13
shRNA Suppression Studies
Human SHROOM3 short hairpin clones (Open Biosystems, Lafayette, CO) were tested for optimal suppression in 293-T cells. The selected GFP-tagged hairpins were used to generate a mammalian VSV pseudotyped lentiviral expression construct. Lentiviral medium was used to infect human podocytes at 33°C. Cells were passaged in puromycin (5 µg/ml)-RPMI 1640 for experiments after 7 days differentiation at 37°C.
Reverse Transcription
For in vitro studies we used Superscript-III (Invitrogen, Life Technologies, Grand Island, NY) with starting total RNA approximately 1000 ng.
Quantitative PCR
SHROOM3 expression was assayed in vitro/in vivo by real-time protein-to-creatinine ratios (PCR) (7500; Applied Biosystems). Amplification curves were analyzed using automated 7500 software platform, via the ΔΔCT method. Human glyceraldehyde phosphate dehydrogenase (GAPDH) was used as endogenous control. Similarly, primers were designed for mouse Gapdh, Nphs1, Podocin, and Synaptopodin (Supplemental Table 1).
Western Blotting
Cells were lysed with a buffer containing 1% NP-40, a protease inhibitor mixture, and tyrosine and serine/threonine phosphorylation and phosphatase inhibitors. Lysates were subjected to immunoblot analysis using polyclonal rabbit anti-SHROOM3 (#SAB3500818; Sigma), anti-V5 tag antibody (A01724; GenScript Inc.), Phospho-SRC family Y416 rabbit mAb (#2101S; Cell Signaling Technology), nonphosphorylated SRC family Y416 mouse mAb (#2102S; Cell Signaling Technology), Fyn rabbit polyclonal antibody (#4023S; Cell Signaling Technology), mouse monoclonal Fyn (#610163; BD Biosciences), phospho-Nephrin (Y1176/1193; #Ab80299; Abcam), total Nephrin (gift from Dr. Lawrence Holzman to J.C.H.), and actin (mouse monoclonal, #A5441; Sigma). Anti-V5 magnetic beads (MBL International) were used for immunoprecipitation. Densitometry was performed on images of Western blots using ImageJ software.
Flow Cytometry
Human podocytes (Scramble or Si-2) were incubated for 20 minutes at 4°C in either annexin buffer (BD-556454) or PBS with 1:10 AnV (BD-550474) and 1:1000 viability dye (65–0863–14; eBioscience). As positive control, podocytes were pretreated with 25% w/v H2O2 for 20 minutes at 4°C before staining.
Mass Spectrometry
PCDEST SHROOM3 and pcDNAcDNAM3 lacZ (control vector) were overexpressed in 293 T cells. Protein lysates after 48 hours transfection were immunoprecipitated with anti–V5-tag, anti-SHROOM3, or control IgG, and run on PAGE gels. Three resultant lanes were sent for mass spectrometry (protein mixture identification by LC/MS; LTQ Orbitrap Velos; ThermoFisher Scientific). Proteins identified in both overexpression and endogenous lanes were selected for further filtering analysis (See Supplemental Material).
In Vivo Studies
Murine Shroom3 Knockdown Model
Tetracycline-responsive, shRNAmir-mediated Shroom3 knockdown mouse strain on the basis of tested shRNA guide sequences was developed with Mirimus Inc., NY. In double-transgenic CAGS-rtTA/Podocin-RTTA/PAX8-RTTA;Shroom3 RNAi mice, shRNAmir-mediated knockdown was driven by the respective universal or tissue-specific promoters and inducible by doxycycline (DOX) feeding.4 Male mice (7–8 week old) were DOX-fed for 6 weeks (600 mg/g DOX chow; Envigo Inc.), and subjected to weekly urine collection (n≥4 mice per group in all data). After 6 or 8 weeks of DOX feeding, kidney tissues were collected for histology, immunofluorescence, RNA isolation for quantitative PCR/RNA-sequencing, protein extraction for Western blotting, and immunoperoxidase. Glomeruli and nonglomerular fractions were extracted using DYNA-bead perfusion.
Adriamycin Injection Study
Eight-week old male Podo-RTTA mice (C57B6/129SVJ/FVB background) and littermates were fed DOX (6 weeks), and were injected with retrobulbar injection (ADR 15 g/kg). DOX was continued. Urine was collected 2-weekly till euthanasia at 8 weeks post injection.
Aged Mice
CAGS-TG mice were aged to 12 months on regular chow. DOX was initiated at 12 months for 12 weeks (n=3 each). Kidney tissue was collected for microscopy and IHC (P57). Morphometry on light microscopic images was performed after thresholding using ImageJ or metamorph software.
Glomerular Morphometry
Tissue Processing.
Glutaraldehyde fixed samples were processed as described.14
Podocyte Number.
The fractionator/dissector method was used to count podocytes. Podocyte nuclei were surrogates for podocytes assuming only one nucleus per podocyte. Using an ultramicrotome, serial 1-µm-thick sections were cut from an epon tissue block. Using Adobe Photoshop, the images from each pair of sections were view together. Podocyte profiles from glomerulus 1 seen in the second section of a pair (the sample section) but not present in the first section (the look-up section) were counted as a Q−s. This counting was repeated for all the pairs of sections from a glomerulus (Supplemental Figure 1E).15,16
Glomerular and Glomerular Component Volumes.
The Cavalieri Principle was used to measure glomerular volume (Supplemental Figure 2).17–19 An average of 7.4 glomeruli was measured per kidney. An average of 841.5 points were counted per kidney. The glomerulus was divided into four components: podocytes, mesangium, capillary lumens and endothelial space, and other. The other component was defined as Bowman’s space, glomerular basement membrane, and nonresolvable areas within the glomerulus, and were not used in glomerular component analysis.
Foot Process Width.
Average foot process width was determined as described before (Supplemental Figure 1G).20,21
RNA-Sequencing
RNA was isolated from glomerular/nonglomerular fractions after homogenization (RNeasy kit; Qiagen). Ribosomal RNA depletion was performed before Poly-A selection to improve quality. Single-ended sequencing with 75 bp read length was carried out on NEXTSEQ (Illumina). The differential analysis between transgenic and nontransgenic (NTG) samples by paired LIMMA was performed to identify significantly dysregulated genes at P value <0.05, which were then subjected to Gene Ontology function and canonical pathway enrichment analysis by Fisher exact test. Genes uniquely and exclusively downregulated in glomerular fraction, but not in nonglomerular fraction were identified and compared via Gene Ontology function and canonical pathway enrichment analysis. Podocyte-enriched genes were similarly compared on the basis of prior data.22 Complete data is available on the Gene Expression Omnibus database (GEO-GSE110092).
Statistical Analyses
Descriptive statistics were used to summarize participants, and compared by chi-squared/Fisher exact test. Spearman correlation was used to determine relationship between SHROOM3 expression, and eGFR (Nephroseq). Multivariable linear regression was performed, with ACR and PCR as outcomes. Predictors were assessed using baseline variables (SPSS version 23; SPSS Statistics).
Study Approval
Institutional review board approval for human subjects in GoCAR was obtained from all sites; informed written consent was taken from all participants (living donors/recipients). All animal studies were in accordance with protocols approved by the Institutional Animal Care and Use Committee at Mount Sinai. The study adheres to the STROBE checklist for cohort studies (see Supplemental Material).
Results
Renal Biopsies Show Dichotomous Associations of Glomerular versus Nonglomerular Shroom3 Expression with CKD
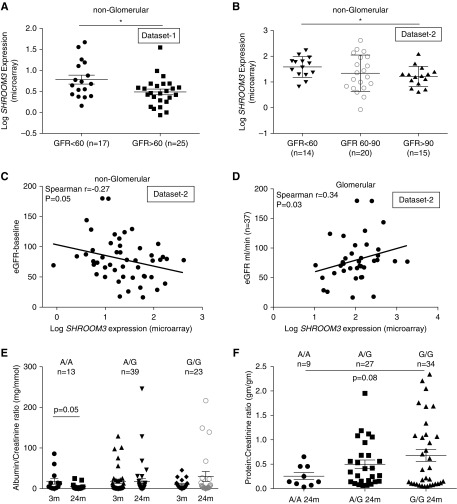
As the rs17319721 enhancer associated with lower GFR but lower albuminuria,11 we hypothesized that SHROOM3 expression in renal compartments would show dichotomous associations with CKD. We used public datasets that reported CKD (by eGFR), and renal SHROOM3 expression (glomerular/nonglomerular fraction data).23,24 In the nonglomerular transcriptome of dataset 1, CKD (eGFR<60 ml/min) was associated with significantly higher SHROOM3 compared with biopsy samples with eGFR>60 ml/min (Figure 1A). Similarly, in dataset 2, nonglomerular mean SHROOM3 in biopsy samples with CKD was significantly higher than those with eGFR>90 ml/min (Figure 1B). We observed an inverse correlation between nonglomerular SHROOM3 and eGFR in dataset 2 (n=49; Figure 1C). These relationships between nonglomerular SHROOM3 and CKD are consistent with CKD-GWAS that identified rs173197211 and the enhancer function of this SNP.4 However, glomerular transcriptome from dataset 2 showed a linear correlation between SHROOM3 and eGFR, suggesting an opposite relationship between glomerular SHROOM3 and eGFR (n=37; Figure 1D). These data suggest potential dichotomous roles of SHROOM3 in distinct renal compartments in CKD.
Figure 1.
Glomerular versus nonglomerular SHROOM3 expression and rs17319721 have dichotomous associations with eGFR and albuminuria in humans. Dot graphs plot SHROOM3 expression (log SHROOM3 expression by microarray) in nonglomerular fraction of (A) dataset 123 and (B) dataset 224 (glomerular and nonglomerular transcriptome) of renal biopsy samples plotted against eGFR (ml/min). Figure shows the correlation lines between eGFR (ml/min) and (C) nonglomerular and (D) glomerular SHROOM3 expression in dataset 2 (Spearman R=−0.27 and 0.35; P=0.05 and 0.03, respectively). (E) Dot plots show serial mean ACR (mg/mmol) at 3 and 24 months in GoCAR Sydney cohort displayed by donor SNP type (paired t-test P=0.05 between 3- and 24-month A/A donors). (F) Dot plots show mean protein-to-creatinine ratios (mg/gm) at 24 month cross-sectionally by donor SNP type (unpaired t test A/A versus G/G =0.08). Dot plots/whiskers=mean/SEM; *P<0.05.
Shroom3 SNP rs17319721 in the Donor Associates with Reduced Albuminuria after First Transplant Year
Because rs17319721 increased SHROOM3 expression in allografts and associated with higher CADI score/lower eGFR,4 we examined the association of donor genotype and proteinuria using two cohorts within GoCAR: GoCAR-Sydney (n=75; 13 A/A, 39 A/G, 23 G/G), with serial albumin-to-creatinine ratios (ACRs); and GoCAR-Sinai (n=70; 9 A/A, 27 A/G, 34 G/G), with protein-to-creatinine ratios, recorded at 2-year visit (Table 1, demographics). The demographics of GoCAR enrollees have been previously published.4,22 Because early proteinuria is confounded,25 we assessed early (3-month) versus late (24-month) albuminuria in GoCAR-Sydney. By 24 months, A/A allografts had the lowest ACRs (paired t test P=0.05; A/A donors, 3-month versus 24-month albuminuria, 18.2±7.3 versus 5.7±2.2 mg/mmol, respectively; NS for A/G or G/G allografts; Figure 1E). In multivariable models (adjusted for recipient/donor characteristics) A/A allografts had lower ACR at 24 months (Table 2). In GoCAR-Sinai, protein-to-creatinine ratios were performed for clinical care at the 2-year visit, A/A allografts had significantly lower protein-to-creatinine ratios in multivariable analyses (Figure 1F, Table 2). These data suggest that donor A-allele has a mild but significant effect attenuating late albuminuria in allografts, despite increased CADI score.
Table 1.
Demographics of GoCAR-Sinai and GoCAR-Sydney cohorts
| Demographics | GoCAR-Sinai (n=70) | GoCAR-Sydney (n=75) |
|---|---|---|
| Donor age, mean±SD [range] | 45.67±14.9 yr [3–68] | 40.77±15.9 yr [14–76] |
| Donor sex (% females) | 24 (34.3) | 36 (48.0) |
| Donor race | ||
| White (W) | 33 (47.1) | 68 (90.7) |
| Black (AA) | 15 (21.4) | 0 (0.0) |
| Hispanic (H) | 15 (21.4) | 0 (0.0) |
| Other (O) | 7 (10.0) | 7 (9.3) |
| Donor type | ||
| Deceased donors | 57 (81.4) | 38 (50.7) |
| Living donors | 13 (18.6) | 37 (49.3) |
| Recipient age, mean±SD [range] | 56.57±12.18 yr [26–83] | 44.21±12.6 yr [19–69] |
| Recipient race | ||
| White (W) | 19 (27.1) | 61 (81.3) |
| Black (AA) | 33 (47.1) | 0 (0.0) |
| Hispanic (H) | 11 (15.7) | 0 (0.0) |
| Other (O) | 7 (10.0) | 14 (18.7) |
| Recipient sex (% females) | 49 (70.0) | 28 (37.3) |
| Recipient ESRD | ||
| Diabetes | 29 (41.4) | 29 (38.7) |
| Hypertension | 24 (34.3) | 21 (28.0) |
| Glomerular disease | 8 (11.4) | 10 (13.3) |
| Others | 9 (12.9) | 15 (20.0) |
| Albuminuria, mean±SEMa | NA | |
| 3-mo versus 24 mo | 18.6±7.3 versus 5.7±2.2 | |
| A/A donors (n=13) | 17.5±4.9 versus 17.2±7.1 | |
| A/G donors (n=39) | 9.7±2.4 versus 30.1±12.1 | |
| G/G donors (n=23) | ||
| Proteinuria, mean±SEMb | NA | |
| 2-yr visit | 0.25±0.21 | |
| A/A donors (n=13) | 0.49±0.46 | |
| A/G donors (n=39) | 0.68±0.71 | |
| G/G donors (n=23) |
Albumin: Creatinine ratio (mg/mol).
Protein: Creatinine ratio (mg/g).
Table 2.
Multivariable regression analysis of donor SNP genotype and proteinuria phenotype
| Covariates | GoCAR-Sydney (n=75) | GoCAR-Sinai (n=70) | ||
|---|---|---|---|---|
| Coefficient | P Value | Coefficient | P Value | |
| Donor characteristics | ||||
| Donor SNP (ref-A/A) | 0.272 | 0.04 | 0.232 | 0.05 |
| Donor status (ref-DD) | −0.044 | 0.72 | 0.117 | 0.33 |
| Donor race (ref-W) | −0.205 | 0.11 | −0.156 | 0.20 |
| Donor sex (ref-F) | 0.111 | 0.38 | 0.093 | 0.44 |
| Recipient characteristics | ||||
| Donor SNP (ref-A/A) | 0.235 | 0.05 | 0.248 | 0.04 |
| ESRD etiology (ref-DM) | 0.178 | 0.13 | −0.038 | 0.77 |
| Recipient race (ref-W) | −0.067 | 0.57 | 0.007 | 0.95 |
| Recipient age | −0.104 | 0.38 | 0.707 | 0.48 |
A/A, A/A genotype; DD, deceased donor recipients; W, white; F, female; DM, diabetes mellitus.
Glomerular and Podocyte-Specific Shroom3 Knockdown Induce Reversible Albuminuria with Podocyte Foot Process Effacement without Podocyte Loss
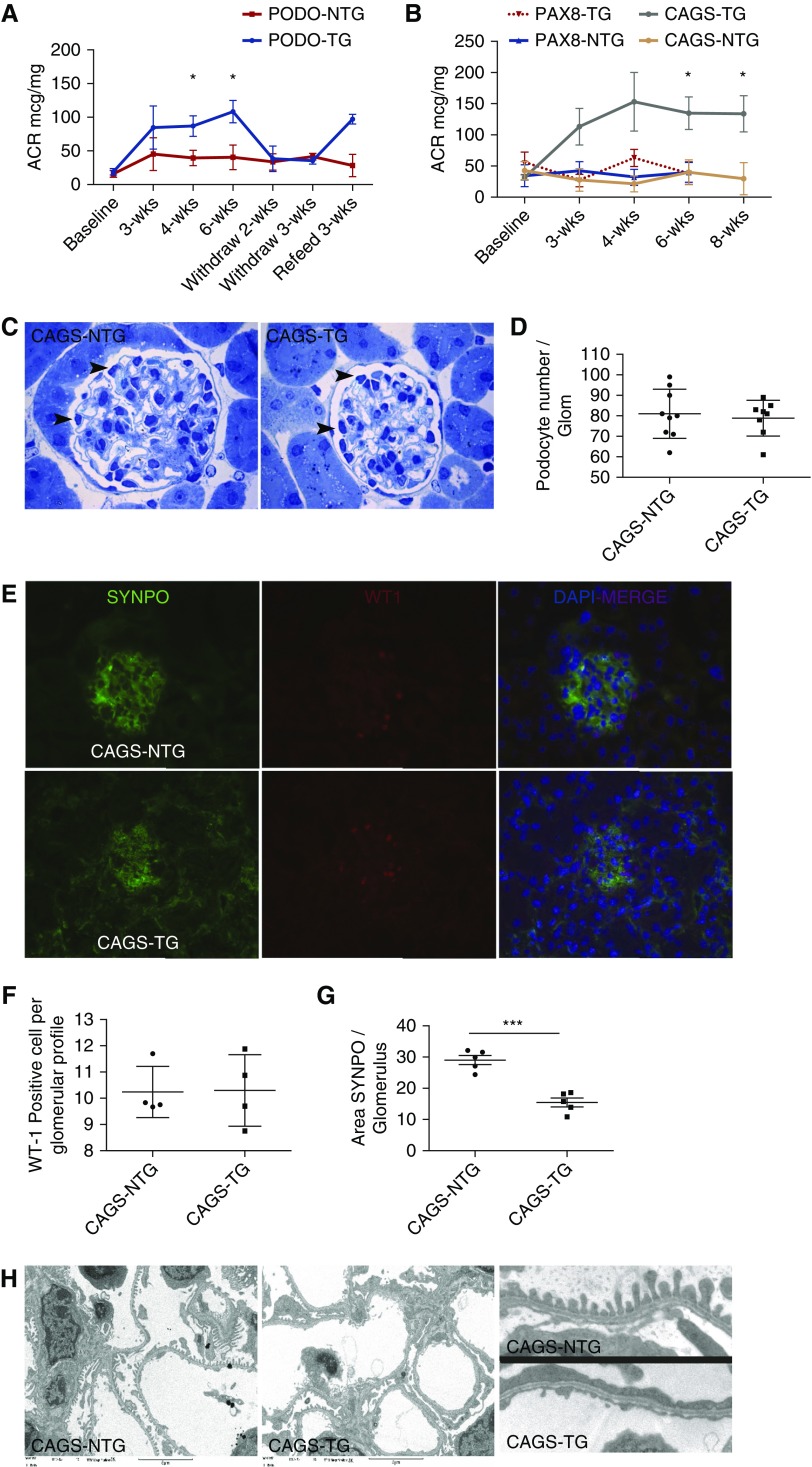
We examined Shroom3 antagonism in adult glomeruli using global- and tissue-specific knockdown mice, as rs17319721 translated into an approximately 1.5-fold increase in SHROOM3 expression in allografts,4 and a milder effect on albuminuria,11 than reported in Shroom3 knockout animals.9,10 We fed adult male double-transgenic CAGS- (global), Podo-, and PAX8-RTTA/Shroom3-ShRNA mice (CAGS-TG, Podo-TG, PAX8-TG), as well as NTG controls with DOX performing urine ACR estimations. Glomerular extracts of CAGS-TG/Podo-TG, but not PAX8-TG showed Shroom3 knockdown (Supplemental Figure 1, A and B), whereas withdrawal of DOX (week 3) restored Shroom3 protein in kidney lysates of CAGS-TG (Supplemental Figure 1C). CAGS- and Podo-TG mice developed significant albuminuria by week 3 compared with respective NTG- or PAX8-TG mice, suggesting a podocyte-specific effect of Shroom3 knockdown (Figure 2, A and B; n=5 mice). Proteinuria reversed in Podo-TG upon DOX withdrawal (>2 weeks), and reappeared on reinitiation (Figure 2A). We examined glomeruli of CAGS-TG at 6 and 8 weeks, compared with CAGS-NTG. We chose BALBc CAGS-TG mice because of their stronger Shroom3 knockdown (Supplemental Figure 1, C versus A) and to avoid strain-based heterogeneity. No loss of podocytes was identified in CAGS-TG mice by fractionator/dissector technique (6 weeks; see Methods; Figure 2,C and D, Supplemental Figure 1E), or by Wt-1/Synpo costaining (8 weeks; Figure 2, E and F). Interestingly we identified a significant decrease in area of Synpo stain in glomeruli from CAGS-TG mice (Figure 2G). No glomerulosclerosis was observed in the CAGS-TG group (Supplemental Figure 1F). Electron microscopy revealed extensive foot process effacement (FPE), with higher mean foot process width in representative CAGS-TG (Figure 2H, Supplemental Figure 1G; Methods).
Figure 2.
Glomerular Shroom3 knockdown causes albuminuria in adult mice with FPE without podocyte loss. (A) Line graphs compares trend lines of serial ACRs in Podo-RTTA mice (Podo-TG) and nontransgenic littermates (Podo-NTG) upon DOX feeding, withdrawal and reinitiation. (B) ACR trend lines in CAGS-TG, CAGS-NTG, PAX8-TG, and PAX8-NTG animals. (C) Representative images of CAGS-NTG and -TG glomeruli with identification of podocytes (black arrowhead) for quantification by the fractionator/dissector technique. (D) Quantification of podocytes/glomerulus (n=8 mice per group; mean±SD). (E) Right to left: representative 40× immunofluorescence images for SYNPO, WT1, and merge (with DAPI), respectively (top row: CAGS NTG-animals, second row: CAGS-TG). (F) Podocyte quantification (n=4 mice per group) by WT1/DAPI-merge nuclei/glomerular profile, and (G) shows quantification of area SYNPO stain/glomerular profile (>30 glomeruli per kidney) (line/whiskers=mean/SD). (H) Right to left: representative electron microscopic images (3000×) of CAGS-NTG and -TG animals; left panel shows sample inlay magnified; Mean foot process width (five glomeruli per mouse) in representative NTG and TG animals were 267 and 441 nm, respectively. *P<0.05; **P<0.01; ***P<0.001.
Glomerular RNA-Sequencing Identifies Downregulated Key Podocyte Signal-Transduction Pathways with Shroom3 Knockdown
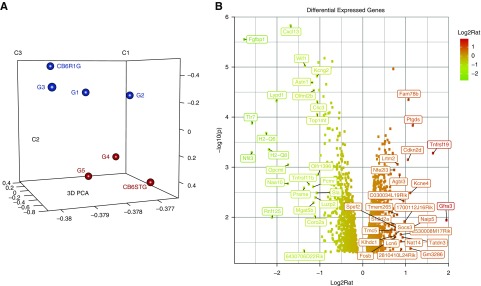
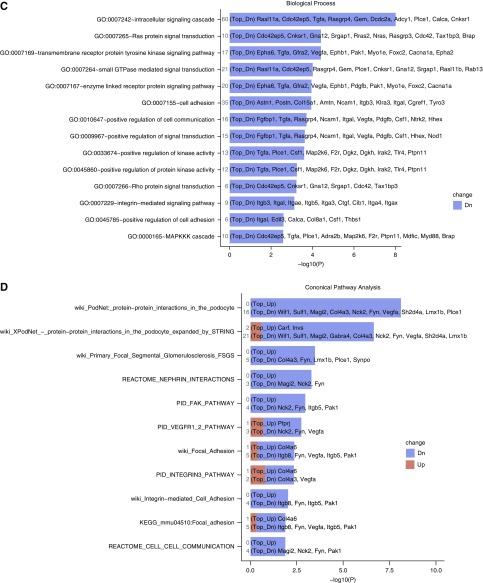
We performed RNA-sequencing on glomerular/nonglomerular fractions (6 weeks DOX). Principal component analysis showed clustering of transgenic versus NTG glomeruli (Figure 3A; one failed QC). Of 1225 and 1102 genes significantly up- and downregulated (DEGs) in glomerular transcriptome between CAGS-TG and -NTG, 877 and 704 DEGs respectively, were unique to glomeruli (Figure 3B; LIMMA test P<0.05; GSE110092). Pathway analysis of unique DEGs showed predominant downregulation of genes involved in tyrosine kinase/Small-GTPase/Integrin signaling, and Actin cytoskeletal regulation (Supplemental Figure 3A). Clustered Gene Ontology analysis was done on 704 downregulated, glomerular-unique DEGS. Key downregulated Gene Ontology terms in CAGS-TG overlapped with pathway analysis (Figure 3C, Supplemental Table 2). Among podocyte-enriched DEGs22 (Supplemental Figure 3B), Synpo downregulation was identified by RNA-sequencing and confirmed by quantitative PCR whereas NPHS1/NPHS2 mRNA-levels were not significantly downregulated by RNA-sequencing or quantitative PCR (Supplemental Figure 1D). Metapathway analysis of podocyte-specific genes revealed predominant downregulation of podocyte signal-transduction pathways (Figure 3D, 12/15 ranked by P value). These data suggest glomerular Shroom3 knockdown induces downregulation of key podocyte signal-transduction pathways implicated in the regulation of actin cytoskeleton.
Figure 3.
Glomerular RNA-sequencing identifies downregulated tyrosine kinase and small GTPase intracellular signaling pathways affecting actin cytoskeleton in podocytes with Shroom3 knockdown. (A) Principal component analysis plot of glomerular transcriptome of CAGSNTG (black dots; n=4) and CAGS-TG (red dots; n=3). (B) Volcano plot of differentially expressed genes between NTG and TG glomeruli unique to the glomerular fraction (877 upregulated and 704 downregulated genes). (C) Bars show relevant clustered Gene Ontology analysis terms of downregulated genes unique to glomerular transcriptome (among top 50 Gene Ontology terms by P-value). (D) Top 12/15 metapathway terms ranked by P-value isolating podocyte-specific genes that were differentially expressed in CAGS-TG versus NTG comparisons, both plotted against negative log(10) P values. Both graphs demonstrate downregulation of key signaling pathways–tyrosine kinase and small GTPases involved in signal transduction in podocytes (G4, G5, and CB6ST=TG group; G1, G2, G3, CB6R1G=NTG group).
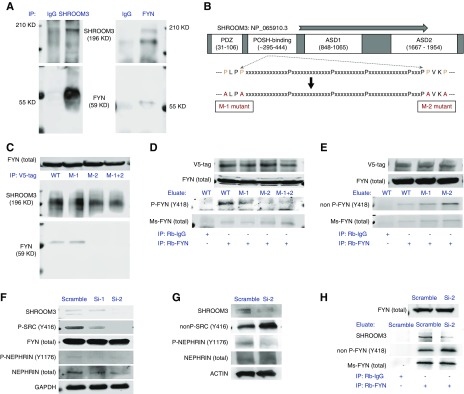
Immunoprecipitation Identifies SHROOM3-FYN Interaction in Podocytes via SH3-Binding Domain
To screen the protein interactome of SHROOM3, we performed mass spectrometry (LC/MS) on 293-Tcell lysates immunoprecipitated with V5- (overexpressed SHROOM3), and Shroom3- (endogenous) antibodies. We identified 287 unique interactions common to endogenous and overexpressed SHROOM3. The top ranked 20 proteins are in Supplemental Table 3. We confirmed ROCK2, ROCK1, and 14–3-3 as binding partners for SHROOM3.7,8 Interestingly, we identified FYN among the top-ranking proteins (Supplemental Table 3). FYN is a nonreceptor tyrosine kinase and activator of several pathways,26 which were downregulated in Shroom3 knockdown glomeruli (Figure 3, C and D). Kinase enrichment analysis (KEA; ENRICHR database),27 showed enrichment for multiple Src-kinases including FYN (Supplemental Figure 4). In podocytes, FYN binds Nephrin, phosphorylating the cytoplasmic tail of Nephrin with subsequent actin cytoskeletal organization by recruiting NCK adapter proteins.28 Hence we investigated whether FYN mediated effects of SHROOM3 knockdown on podocytes.
We confirmed the interaction of endogenous FYN and SHROOM3 by immunoprecipitation in human podocytes (Figure 4A). FYN has Src-homology-domain 3- (SH3) and SH2 domains responsible for interactions with complementary binding-domain containing proteins.28,29 Shroom3 binds Posh (Plenty of SH3-domains protein)30; where a conserved Shroom3 region (peptide approximately 295–444) was critical to Posh-binding. LC/MS-KEA data also showed enrichment of multiple kinases with SH3 domains (Supplemental Figure 4). Therefore, we examined this proline-rich region in SHROOM3 (Refseq-NP_065910.3) for SH3-binding sites, and identified two -PxxP- loci, 333–37 and 445–8 (Figure 4B). SHROOM3-CDNA construct4 was mutated at these sites individually (P➔A substitutions) generating SHROOM3-M1, -M2, and M1+2 constructs (Figure 4B). SHROOM3/mutants were overexpressed in 293-T cells followed by immunoprecipitation with V5 beads. Immunoblotting for FYN showed site 445–8 (i.e., M2 mutation) is the critical FYN-binding site of SHROOM3 (Figure 4C). These data confirm the interaction of endogenous FYN and SHROOM3 in podocytes via SH3-SH3 binding domains, respectively.
Figure 4.
SHROOM3 interacts with Src kinase FYN in podocytes in vitro, at SH3-binding domain, and regulates FYN activation by phosphorylation. (A) Immunoprecipitation of human podocyte cell lysates with anti-SHROOM3 and anti-FYN antibodies, run on PAGE, with probing for FYN and SHROOM3, respectively. Figure shows representative immunoblots. (B) Figure shows SHROOM3 protein with consensus domains, and putative POSH-binding conserved domain. This Proline rich sequence was mutated at two identified –PxxP- loci generating -AxxA- sequences, mutants M1, M2, and M1+2. (C–E) Lysates from 293T cells overexpressing SHROOM3, M1, M2, or M1+2 mutants were immunoprecipitated with anti-V5 tag, or Rb-FYN antibodies. Representative Western blots of cell lysates immunoblotted for (C–E) Rb-FYN, (C and D) V5 (as loading controls), and immunoblots of eluates with (D and E) Ms-FYN, (D) Phospho-SRC (Y416), or (E) nonphosphoY416 SRC antibodies, respectively, are shown. (F and G) Human podocyte cell lines were stably infected with control (Scramble) or Shroom3 knockdown (Si-1, Si-2) puromycin-selectable lentiviruses. Representative Western blots from differentiated Scramble, Si-1, Si-2 podocyte lysates probed for (F) SHROOM3, FYN, phospho SRC family (Y416), phospho-NPHS1 (Y1176/Y1193), total NPHS1, GAPDH (G) SHROOM3, nonphospho SRC (Y416), phospho-NPHS1 (Y1176/Y1193), total NPHS1, ACTIN, respectively from independent experiments are shown. (H) Lysates from Scramble- and Si-2 podocyte lines were immunoprecipitated with Rb-IgG, or Rb-FYN. Immunoblots for Rb-Fyn (total lysate: top row), and SHROOM3, nonphospho FYN (Y418), and total FYN (eluates: bottom three rows) are shown. Ms, mouse; Rb, rabbit.
SHROOM3-FYN Interaction Regulates FYN Activation and Phosphorylation of Cytoplasmic Tail of Nephrin
We interrogated FYN phosphorylation (Y418, activation) with/without SHROOM3-FYN interaction. Total-FYN immunoprecipitation eluates from 293 cell lysates overexpressing SHROOM3 or mutants were probed for activation (using generic Y416 antibodies). In eluates, from M2 to M1+2 mutant lysates, i.e., without SHROOM3-FYN interaction, phospho-Y418-FYN was inhibited compared with SHROOM3- and M1-mutants (Figure 4D). M2 mutant FYN eluates had increased nonphospho-Y418-FYN (Figure 4E).
To evaluate FYN activation in podocytes without SHROOM3, we created stable SHROOM3 knockdown human podocyte lines (Si–1 and 2). Si-2 demonstrated greater SHROOM3 knockdown of both isoforms [4F]. Here, immunoblotting identified reduced phospho-Y416 and increased nonphospho-Y416 with the respective total-SRC-kinase antibodies in Si-2 versus Scramble lysates (Figure 4, F and G, respectively). FYN phosphorylates tyrosine residues located in conserved Nck-binding YDxV motifs within the cytoplasmic tail of NPHS1 (Y1176/Y1193).31 Phospho-NPHS1 was significantly inhibited in Si-1 and 2, downstream of FYN (Figure 4, F and G). Total FYN immunoprecipitation eluates from Scramble- and Si-2 lysates showed significantly increased nonphospho-Y418-FYN in Si-2 versus Scramble (Figure 4H). Hence, SHROOM3-FYN interaction in podocytes regulates FYN activation, and phosphorylation of NPHS1.
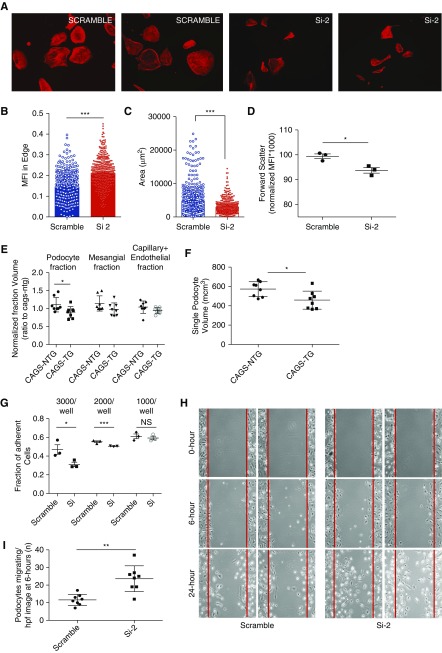
SHROOM3 Knockdown Alters Podocyte Morphology and Actin Cytoskeleton In Vitro
We examined podocyte cytoskeleton by phalloidin F-actin staining in Si-2 and Scramble cells,9,31,32 and identified altered actin bundle distribution with greater marginal staining in Si-2 cells (Figure 5, A and B). Interestingly significantly reduced podocyte cellular area was seen in Si-2 cells (Figure 5C). Flow cytometry of differentiated podocytes further identified reduced forward-scatter mean fluorescence intensity implying reduced cell size in Si-2 versus Scramble (Figure 5D, Supplemental Figure 5, A and B). Correspondingly, in vivo, significant reductions in mean volume of the podocyte fraction/glomerulus, and single podocyte volume were seen in CAGS-TG (n=7 glomeruli per mouse; n=8 mice; Figure 5, E and F), whereas nonpodocyte glomerular fractions were not significant (see Methods). The differences in cell size were not because of diminished cell viability, increased cell death, or increased apoptosis (Supplemental Figure 5, C–G).
Figure 5.
SHROOM3 knockdown alters podocyte actin cytoskeletal organization, morphology, adhesion and migration. (A) Right to left: representative immunofluorescence images (20×) of phalloidin F-actin cytoskeletal stain of Scramble versus Si-2 podocyte cell lines (labeled). Dot plots summarize (B) ACTIN bundle intensity at cellular margins and (C) cell area of phalloidin-stained podocytes (n=4 experiments; 150 cells per experiment). (D) Dot plots show mean flow cytometric forward scatter (size) of trypsinized cell suspensions of podocytes normalized to Scramble podocytes (n=3 experiments; see Supplemental Figure 5, A and B). (E) Graph compares glomerular component volumes (podocyte, mesangial, and capillary+endothelial fractions), and (F) average single podocyte volume, between CAGS-NTG and CAGS-TG mice (n=8 mice; 6 weeks DOX). (G) Scramble and Si-2 podocyte cell lines were differentiated and plated on laminin-coated 96-well plates at 1000, 2000, and 3000 cells/well densities (n=3 sets for each density). Bar graphs compare proportion of adherent podocytes: total podocytes in each plating density at 1 hour by turbidimetry (crystal violet method). (H) Differentiated Scramble and Si-2 podocytes were plated in 10-cm dishes. Scratches were made on fully confluent plates using sterile pipette tips. Panels show representative phase-contrast images (4×) of Scramble and Si-2 conditions at 0, 6, and 24 hours. (I) Line dot graphs compare number of cells inside scratch margins of eight streaks in duplicate plates at 6 hours (line dot graphs/whiskers=mean/SD; *unpaired t test P<0.05; **P<0.01; ***P<0.001.
To examine podocyte adhesion, differentiated scramble/Si-2 cells were plated in laminin-coated 96-well plates. We observed significantly reduced adhesion in Si-2 at 1-hour (Figure 5G). Using Scratch assay,33 Si-2 cells showed increased podocyte migration versus Scramble (Figure 5, H and I), corresponding to FPE identified in vivo.
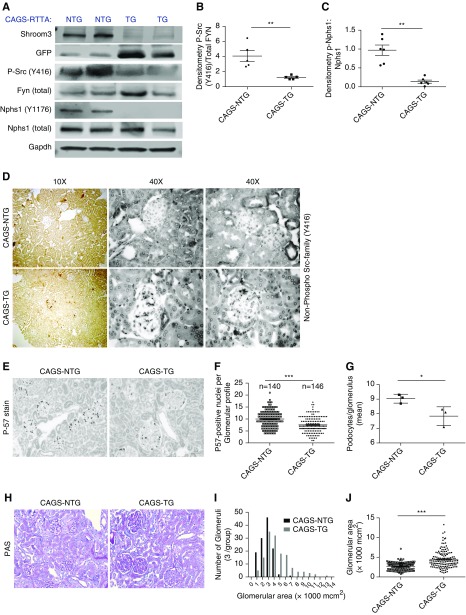
Shroom3 Knockdown Inhibits Fyn Activation and Nphs1-Phosphorylation In Vivo
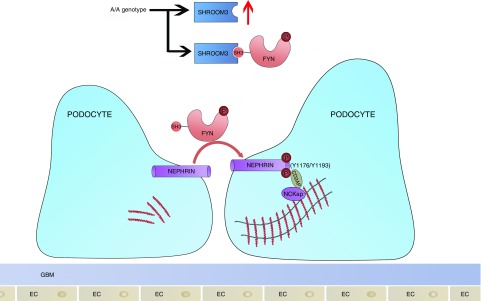
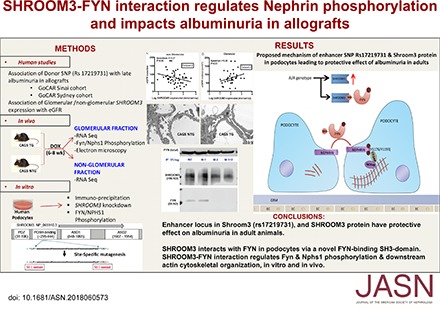
Glomerular lysates from CAGS-TG and -NTG mice were studied after the development of stable proteinuria (6 weeks DOX). Here, Shroom3-specific ShrRNA with DOX feeding is cotranscribed with GFP in cells with ShRNA expression.4 Human FYN Y418 corresponds to Fyn Y423 in mice.34 Phospho-Y416-Src (Figure 6A) and Nphs1-phosphorylation (Y1176/Y1193) were inhibited, whereas total Nphs1 was unchanged (Figure 6A, Supplemental Figure 6A) with Shroom3 knockdown in glomerular extracts, with significantly reduced phospho-Src (Y416):Fyn (Figure 6B) and p-Nphs1:Nphs1 ratios (Figure 6C). Glomerular cells of CAGS-TG mice also showed increased nonphospho-Y416 staining compared with CAGS-NTG by IHC (Figure 6D). Together, our data confirm that SHROOM3 interacts with FYN via SH3-binding domain (445–8), and regulating FYN activation, and downstream signaling including NPHS1phosphorylation (Y1176/Y1193) and maintenance of podocyte actin cytoskeleton and phenotype. In this context, the presence of the enhancer A-allele would be beneficial in podocytes (summary in Figure 7).
Figure 6.
In vivo glomerular Shroom3 knockdown inhibits Fyn phosphorylation and downstream phosphorylation of cytoplasmic tail of Nephrin. CAGS-NTG and TG littermate male mice were fed DOX (6 weeks). (A) Representative Western blots (n=2 each group shown) demonstrate immunoblotting for Shroom3, TGFP, Phospho Src (Y416), total Fyn, Phospho Nphs1 (Y1176/Y1193), Nphs1, and Gapdh. (B) Bar graphs show densitometry of Phospho Src Y416: total Fyn and (C) Phospho Nphs1:total Nphs1 between CAGS TG and NTG mice (n≥5 sets). (D) Representative 10× (left: two panels) and 40× (right panels) photomicrographs from two animals of each group, show immunoperoxidase stain for nonphospho SRC (Y416). CAGS NTG and TG mice were aged approximately 1 year (n=3 mice each), then DOX-fed (12 weeks). (E) Representative photomicrographs (20×) of immunoperoxidase for P57-positive nuclei (podocyte nuclei) between CAGS NTG and TG mice, and dot graphs quantify podocyte number/glomerular profile, depicted as (F) number of profiles counted per experimental condition, and (G) per mouse. (H) Representative PAS stained sections (20×) showing glomerular enlargement with mesangial sclerosis in CAGS-TG mice compared with NTG. (I) Histogram shows distribution of area of individual glomeruli (×1000 mcm2) in CAGS NTG (dark grey) and TG (light grey) (mean of 47.67 glomerular profiles/mouse). (J) Dot graphs compare mean area/individual glomerular profile in CAGS NTG versus TG (bar graphs/whiskers=mean/SEM; *unpaired t test P<0.05); **P<0.01; ***P<0.001.
Figure 7.
Cartoon summarizes putative mechanism of protective effect of the enhancer locus in Shroom3 (rs17219731) and SHROOM3 protein on albuminuria in adults. Homozygosity for the CKD-associated allele (A/A) associates with higher SHROOM3 expression, preserved interaction with FYN in podocytes (via SH3-binding domain), activation of FYN kinase (Y418 phosphorylation), downstream Nephrin tail phosphorylation at NCK-adapter protein (NCKap) docking sites, and actin cytoskeletal stabilization. Depletion of SHROOM3 causes cytoskeletal disarray by inhibition of Fyn activation and nephrin phosphorylation.
To examine whether Shroom3 knockdown increased susceptibility to glomerular injury, we performed adriamycin injections in Podo-TG mice (6 weeks DOX) versus littermates. To avoid confounding from tubular Shroom3 knockdown, we used Podo-TG mice albeit in a hybrid background with littermate controls (n=5 each). Proteinuria was similar in Podo-TG and NTG without FSGS lesions in either group (Supplemental Figure 6, B and C). Because Fyn knockout mice show FPE without FSGS up to 1 year of age,28 and heterozygous Shroom3 knockout mice showed FSGS at 1 year of age despite FPE,10 we aged CAGS-TG and -NTG mice (1 year), and DOX fed these mice for 8 and 12 weeks. At 8 weeks, CAGS-TG mice showed mesangial expansion and protein reabsorption droplets in tubules versus aged controls (n=4; Supplemental Figure 6D). This was distinct from young mice (Supplemental Figure 1F). After 12 weeks, aged CAGS-TG mice (n=3) showed no weight loss (Supplemental Figure 6G) but significant podocyte loss by P57-positive nuclear staining (Figure 6, E–G), and increased glomerular area with mesangial expansion (Figure 6, H–J), supporting an age-dependent phenotype of Shroom3 knockdown. Interestingly, nonglomerular Shroom3 protein was similar in aged CAGS-TG and -NTG mice with glomerulosclerosis, whereas simultaneous glomerular Shroom3 was knocked down (Supplemental Figure 6, E and F). This suggests upregulation of tubular Shroom3 with glomerulosclerosis. These data from the nonglomerular compartment in FSGS mice are consistent with dichotomous expression patterns seen in glomerular/nonglomerular transcriptomes in human CKD biopsy samples.
Discussion
We previously identified that allograft SHROOM3 expression associated with subsequent renal allograft fibrosis, whereas tubular Shroom3 knockdown reduced fibrosis. We show here the interesting divergent associations of SHROOM3 expression within glomerular/nonglomerular compartments with CKD, as well as the corresponding dichotomous associations of the enhancer A-allele with eGFR and albuminuria phenotypes in allografts. In adult glomeruli, Shroom3 knockdown caused FPE without podocyte loss in the short term. We identified the interaction of the Src-kinase FYN with SHROOM3 via SH3-SH3 binding sites in podocytes, which regulates FYN activation, Nephrin phosphorylation, and actin cytoskeletal organization. In vitro, these corresponded to altered podocyte morphology, adhesion, and migration. Aside from revealing new biology regarding Shroom3 and FYN, these data are an essential first step before the design of therapeutics in humans for renal fibrosis by inhibiting SHROOM3.
Prior data reported severe glomerular phenotypes in homozygous Shroom3 knockout mice from inhibited Shroom3-Rock binding via the ASD2-domain, and in FHH rats via the ASD1-domain.9,10 Notably, heterozygous mice with early FPE developed FSGS only by 1 year. The mechanistic basis for the different phenotypes between homo- and heterozygotes was unexplained. In our study, downregulation of rho-signaling pathways (Gene Ontology analysis) and Synpo (not Nphs1), and increased podocyte motility (implying rho-Rac1 imbalance)35,36 are all consistent with Rock inhibition in podocytes of CAGS-TG mice. However, Rock inhibitors are reported to reduce albuminuria, which is inconsistent with the increased albuminuria in CAGS-TG mice, implying a Rock-independent effect of Shroom3 on podocytes.37–39 Thus, the extent and timing of Shroom3 deficiency could decide the glomerular phenotype, with developmental complete knockout (knockout mice or FHH rats) showing early FSGS via ASD2- or ASD1-domain functions, respectively, whereas heterozygotes or CAGS-TG mice with incomplete Shroom3 deficiency show an age-dependent milder phenotype related to deficient Fyn-binding function.
We reported that in tubular cells, facilitation of TGF-β signaling with excess SHROOM3 was abrogated by Rock inhibitors, suggesting that the profibrotic effect may be ASD2-domain–dependent, and distinct from Fyn-binding site. Hence, the specific roles of the distinct protein binding domains of SHROOM3 in proteinuria, as well as in the nonglomerular compartment, should be examined in future work by designing specific small molecule antagonists. Designing domain-specific small molecule antagonists will require complete crystallographic Shroom3 structure including binding pockets to FYN and ROCK. Such inhibitors using homology modeling without competing domain effects have been designed before.40,41 Additionally, Shroom3-Rock interaction is essential for Rock activation in other epithelia,6,7 and off-target effects will need to be calibrated against potential antifibrotic benefits. Hence tissue-specific delivery systems such as biodegradable nanoparticle attachment will need to be considered.42,43
Among proteinuric kidney diseases, inhibited Fyn-phosphorylation has been associated with human minimal change disease (MCD),44,45 where irreversible renal dysfunction is uncommon.46 Fyn knockout mice also showed mild albuminuria with FPE without FSGS,28 distinct from phosphorylation-deficient nephrin mice.31 These data may suggest that aside from inhibition of Nephrin-phosphorylation, impaired Fyn-activation may affect podocytes by other mechanisms, some of which may indeed be protective. An interesting observation in CAGS-TG was the reduced podocyte volume. Fyn responds to cellular volume changes by activation or inhibition.47 Whether changes in podocyte volume observed with Shroom3 knockdown are fyn dependent or independent and/or contribute to the milder phenotype observed when Fyn activation is disrupted, and whether this interaction plays a role in human proteinuric kidney disease, need to be examined further. It must be simultaneously noted that Fyn hypo-phosphorylation may be an epiphenomenon in human MCD, and small scale GWAS studies in pediatric MCD have not identified susceptibility loci within the Fyn locus.48
The GWAS associations of rs1731921 with CKD1 and lower albuminuria,11 Nephroseq, and our data together indicate the association of SHROOM3 expression with the A-allele, and in turn, its opposing associations with albuminuria and eGFR. Cumulatively, these provide mechanistic basis for the associations of the A-allele with both phenotypes in humans. Our data also confirms the reported interaction of Shroom3 with 14–3-3 (Supplemental Table 3).8 Cathepsin-L–dependent cleavage of Synpo depends on 14–3-3 binding,49 and decreased Synpo protein in CAGS-TG mice may be related to impaired Shroom3 and 14–3-3 interaction. However decreased Synpo mRNA levels likely result from inhibited ROCK action and not 14–3-3 binding.35
Our human data are limited by small sample sizes and differing urine protein measurements, although reduced proteinuria was consistent in both cohorts and in a larger GWAS study.11 We used late albuminuria (24 months) to identify association of rs17319721 with proteinuria because early post-transplant albuminuria (<6 months) is confounded by early events.25 Interestingly, the odds ratio of CKD in GWAS (1.07 [95% confidence intervals (95% CI), 1.00 to 1.15])1 associated with the risk allele was lower than the odds ratio for high CADI score (1.9 [95% confidence intervals (95% CI), 1.10 to 3.59])4. The accelerated phenotype in allografts may be from alloimmunity, immunosuppression, or two-kidney to one-kidney transition, which should be investigated in uninephrectomy or transplant models. The mechanism for age-dependent effect of glomerular Shroom3 knockdown is not explained in our data and needs further study. Fyn was downregulated (approximately 1.4-fold) by RNA-sequencing (Figure 3B), although no significant differences in Fyn protein could be identified in glomeruli in vivo (Figure 6A), a finding we cannot fully explain. FYN and SHROOM3 also correlated in glomerular transcriptome from Nephroseq dataset 2 (Spearman r=0.44; P<0.01). In Nephroseq, these findings could relay the association of both FYN and SHROOM3 with podocyte number, although CAGS-TG mice showed no podocytopenia.
In summary, we describe the proteinuria phenotype from Shroom3 knockdown in adult glomeruli, and describe a novel protein-protein interaction with FYN, which explains this phenotype. Our work ascribes mechanism to the intronic CKD-associated enhancer locus explaining its dichotomous association with albuminuria and renal function in humans.
Disclosures
None
Supplementary Material
Acknowledgments
Research/study design: B.M., J.C.H., and M.C.M.; experimentation: C.W., N.P., R.L., A.C., K.B., F.G., P.C., J.W., and M.C.M.; clinical data: M.K., K.K., P.J.O., B.M., and M.C.M.; analyzing data: Z.Y., W.Z., M.F., T.L., F.S., A.C., J.M.B., J.C., K.B., and M.C.M.; providing reagents: L.K., J.W., K.N.C., and B.D.; manuscript: B.M., J.C.H., and M.C.M. All authors contributed to editing of manuscript.
We thank the shared microscopy research facility and Dr. Richard Gordon of the electron microscopy core at Icahn School of medicine at Mount Sinai, as well as Electron Microscopy Facility at the University of Minnesota Imaging Center. Dr. Jenny Xiang at The Genomics Core Research Facility at Weill-Cornell School of Medicine, New York. We acknowledge critical feedback from Prof Detlef Schondorff and Prof. Peter Heeger during different stages of this work and manuscript. We acknowledge Dr. Lawrence Holzman for the gift of Nphs1 antibody. M.C.M. would like to acknowledge philanthropy from Nina and Homer Eaton, and Pablo Legorreta.
This work was supported by American Heart Association grant 15SDG25870018 (to M.C.M.) and National Institutes of Health grant RO1 DK102420 (to B.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018060573/-/DCSupplemental.
References
- 1.Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, et al.: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böger CA, Gorski M, Li M, Hoffmann MM, Huang C, Yang Q, et al.: CKDGen Consortium : Association of eGFR-related loci identified by GWAS with incident CKD and ESRD. PLoS Genet 7: e1002292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, et al.: SKIPOGH team : Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med 19: 1655–1660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon MC, Chuang PY, Li Z, Wei C, Zhang W, Luan Y, et al.: Intronic locus determines SHROOM3 expression and potentiates renal allograft fibrosis. J Clin Invest 125: 208–221, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshmukh HA, Palmer CN, Morris AD, Colhoun HM: Investigation of known estimated glomerular filtration rate loci in patients with type 2 diabetes. Diabet Med 30: 1230–1235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrand JD, Soriano P: Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell 99: 485–497, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Nishimura T, Takeichi M: Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development 135: 1493–1502, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Prokop JW, Yeo NC, Ottmann C, Chhetri SB, Florus KL, Ross EJ, et al.: Characterization of coding/noncoding variants for SHROOM3 in patients with CKD. J Am Soc Nephrol 29: 1525–1535, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo NC, O’Meara CC, Bonomo JA, Veth KN, Tomar R, Flister MJ, et al.: Shroom3 contributes to the maintenance of the glomerular filtration barrier integrity. Genome Res 25: 57–65, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalili H, Sull A, Sarin S, Boivin FJ, Halabi R, Svajger B, et al.: Developmental origins for kidney disease due to Shroom3 deficiency. J Am Soc Nephrol 27: 2965–2973, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis JW, Chen MH, Foster MC, Liu CT, Larson MG, de Boer I, et al.: CKDGen Consortium; CARe Renal Consortium : Validated SNPs for eGFR and their associations with albuminuria. Hum Mol Genet 21: 3293–3298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al.: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K, et al.: Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest 124: 1098–1113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartzman M, Reginensi A, Wong JS, Basgen JM, Meliambro K, Nicholas SB, et al.: Podocyte-specific deletion of yes-associated protein causes FSGS and progressive renal failure. J Am Soc Nephrol 27: 216–226, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai XY, Basgen JM: Podocyte number in the maturing rat kidney. Am J Nephrol 33: 91–96, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyengaard JR: Stereologic methods and their application in kidney research. J Am Soc Nephrol 10: 1100–1123, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Gundersen HJ, Jensen EB: The efficiency of systematic sampling in stereology and its prediction. J Microsc 147: 229–263, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Basgen JM, Sobin C: Early chronic low-level lead exposure produces glomerular hypertrophy in young C57BL/6J mice. Toxicol Lett 225: 48–56, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffes MW, Brown DM, Basgen JM, Mauer SM: Amelioration of mesangial volume and surface alterations following islet transplantation in diabetic rats. Diabetes 29: 509–515, 1980 [DOI] [PubMed] [Google Scholar]
- 20.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M: Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes 56: 2155–2160, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Gundersen HJ: Estimators of the number of objects per area unbiased by edge effects. Microsc Acta 81: 107–117, 1978 [PubMed] [Google Scholar]
- 22.Fu J, Wei C, Lee K, Zhang W, He W, Chuang P, et al.: Comparison of glomerular and podocyte mRNA profiles in streptozotocin-induced diabetes. J Am Soc Nephrol 27: 1006–1014, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, et al.: ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium : Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 7: 316ra193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sampson MG, Robertson CC, Martini S, Mariani LH, Lemley KV, Gillies CE, et al.: Nephrotic Syndrome Study Network : Integrative genomics identifies novel associations with APOL1 risk genotypes in black NEPTUNE subjects. J Am Soc Nephrol 27: 814–823, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naesens M, Lerut E, Emonds MP, Herelixka A, Evenepoel P, Claes K, et al.: Proteinuria as a noninvasive marker for renal allograft histology and failure: An observational cohort study. J Am Soc Nephrol 27: 281–292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas SM, Brugge JS: Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Lachmann A, Ma’ayan A: KEA: Kinase enrichment analysis. Bioinformatics 25: 684–686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, et al.: Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor J, Chung KH, Figueroa C, Zurawski J, Dickson HM, Brace EJ, et al.: The scaffold protein POSH regulates axon outgrowth. Mol Biol Cell 19: 5181–5192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.New LA, Martin CE, Scott RP, Platt MJ, Keyvani Chahi A, Stringer CD, et al.: Nephrin tyrosine phosphorylation is required to stabilize and restore podocyte foot process architecture. J Am Soc Nephrol 27: 2422–2435, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verma R, Venkatareddy M, Kalinowski A, Patel SR, Salant DJ, Garg P: Shp2 associates with and enhances nephrin tyrosine phosphorylation and is necessary for foot process spreading in mouse models of podocyte injury. Mol Cell Biol 36: 596–614, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HW, Khan SQ, Faridi MH, Wei C, Tardi NJ, Altintas MM, et al.: A podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol 26: 2741–2752, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senis YA, Mazharian A, Mori J: Src family kinases: At the forefront of platelet activation. Blood 124: 2013–2024, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Ellis MJ, Gomez JA, Eisner W, Fennell W, Howell DN, et al.: Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int 81: 1075–1085, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blattner SM, Hodgin JB, Nishio M, Wylie SA, Saha J, Soofi AA, et al.: Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int 84: 920–930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gojo A, Utsunomiya K, Taniguchi K, Yokota T, Ishizawa S, Kanazawa Y, et al.: The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur J Pharmacol 568: 242–247, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Matoba K, Kawanami D, Okada R, Tsukamoto M, Kinoshita J, Ito T, et al.: Rho-kinase inhibition prevents the progression of diabetic nephropathy by downregulating hypoxia-inducible factor 1α. Kidney Int 84: 545–554, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Kolavennu V, Zeng L, Peng H, Wang Y, Danesh FR: Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes 57: 714–723, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Cichonska A, Ravikumar B, Parri E, Timonen S, Pahikkala T, Airola A, et al.: Computational-experimental approach to drug-target interaction mapping: A case study on kinase inhibitors. PLOS Comput Biol 13: e1005678, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jelić D, Mildner B, Kostrun S, Nujić K, Verbanac D, Culić O, et al.: Homology modeling of human Fyn kinase structure: Discovery of rosmarinic acid as a new Fyn kinase inhibitor and in silico study of its possible binding modes. J Med Chem 50: 1090–1100, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Alsaggar M, Liu D: Organ-based drug delivery. J Drug Target 26: 385–397, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Tuffin G, Waelti E, Huwyler J, Hammer C, Marti HP: Immunoliposome targeting to mesangial cells: A promising strategy for specific drug delivery to the kidney. J Am Soc Nephrol 16: 3295–3305, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Zhang SY, Kamal M, Dahan K, Pawlak A, Ory V, Desvaux D, et al.: c-mip impairs podocyte proximal signaling and induces heavy proteinuria. Sci Signal 3: ra39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Audard V, Zhang SY, Copie-Bergman C, Rucker-Martin C, Ory V, Candelier M, et al.: Occurrence of minimal change nephrotic syndrome in classical Hodgkin lymphoma is closely related to the induction of c-mip in Hodgkin-Reed Sternberg cells and podocytes. Blood 115: 3756–3762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vivarelli M, Massella L, Ruggiero B, Emma F: Minimal change disease. Clin J Am Soc Nephrol 12: 332–345, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapus A, Szászi K, Sun J, Rizoli S, Rotstein OD: Cell shrinkage regulates Src kinases and induces tyrosine phosphorylation of cortactin, independent of the osmotic regulation of Na+/H+ exchangers. J Biol Chem 274: 8093–8102, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Debiec H, Dossier C, Letouzé E, Gillies CE, Vivarelli M, Putler RK, et al.: Transethnic, genome-wide analysis reveals immune-related risk alleles and phenotypic correlates in pediatric steroid-sensitive nephrotic syndrome. J Am Soc Nephrol 29: 2000–2013, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buvall L, Wallentin H, Sieber J, Andreeva S, Choi HY, Mundel P, et al.: Synaptopodin is a coincidence detector of tyrosine versus serine/threonine phosphorylation for the modulation of rho protein crosstalk in podocytes. J Am Soc Nephrol 28: 837–851, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.