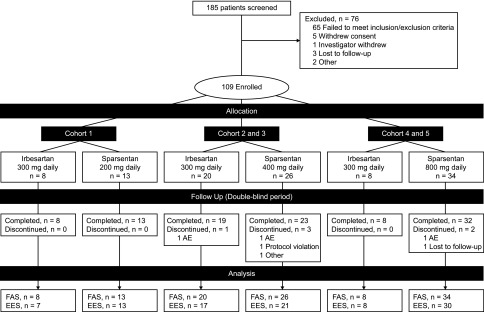
Figure 1.
Patient disposition for the double-blind study period. The prespecified plan for allocation of patients to dose cohorts was 20–40–40 for 200–400–800 mg dose cohorts. The EES population included patients who received at least one dose of study drug and had both baseline and week 8 UP/C assessments. Some patients received half of the assigned nominal dose owing to body wt≤50 kg. AE, adverse event; EES, efficacy evaluable set; FAS, full analysis set.