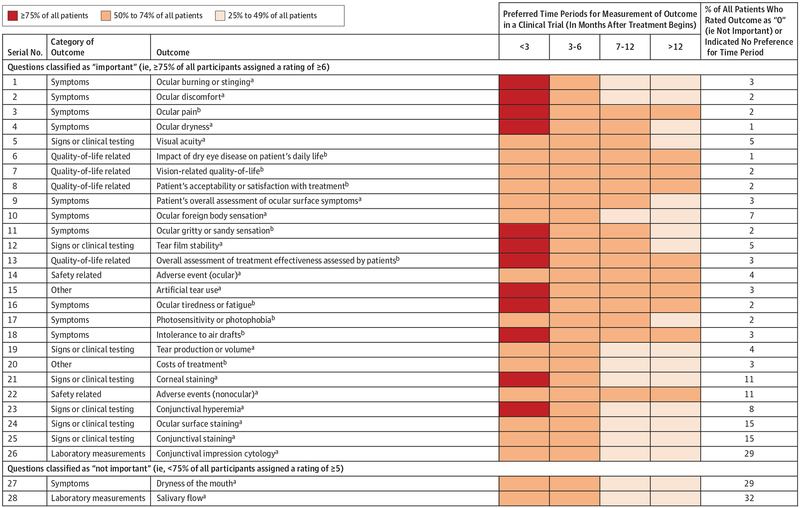
Figure 4. Preferred Outcome Measurement Periods for All 28 Outcomes Rated in Round 2 of the Delphi Survey.
aFor the 18 popular outcomes in existing research (ocular burning or stinging; ocular discomfort; ocular dryness; visual acuity; patient’s overall assessment of ocular surface symptoms; ocular foreign body sensation; tear film stability; ocular adverse events; artificial tear use; tear production or volume; corneal staining; nonocular adverse events; conjunctival hyperemia; ocular surface staining; conjunctival staining; conjunctival impression cytology; dryness of the mouth; and salivary flow), we obtained preferred measurement periods during Delphi round 1 (n = 622 patients). For each outcome, we allowed patients to indicate multiple measurement periods.
bFor the 10 unpopular outcomes in existing research (ocular pain; influence of dry eye disease on patient’s daily life; vision-related quality of life; patient’s acceptability or satisfaction with treatment; ocular gritty or sandy sensation; overall assessment of treatment effectiveness assessed by patients; ocular tiredness or fatigue; photosensitivity or photophobia; intolerance to air drafts; and treatment cost), we obtained preferred measurement periods during Delphi round 2 (n = 420 patients). For each outcome, we allowed patients to indicate multiple measurement periods.