Abstract
Background
Deep nuclear gray matter injury in neonatal hypoxic-ischemic encephalopathy (HIE) is associated with worse neurodevelopmental outcomes. We previously published a qualitative MRI injury scoring system utilizing serial T1-weighted, T2-weighted and diffusion-weighted imaging (DWI), weighted for deep nuclear gray matter injury.
Objectives
To establish the validity of the MRI scoring system with neurodevelopmental outcome at 18–24 months.
Materials and methods
MRI scans from neonates with moderate to severe HIE treated with therapeutic hypothermia were evaluated. Signal abnormality was scored on T1-weighted, T2-weighted and DWI sequences and assessed using an established system in five regions: (a) subcortical: caudate nucleus, globus pallidus and putamen, thalamus and the posterior limb of the internal capsule; (b) white matter; (c) cortex,(d) cerebellum and (e) brainstem. MRI injury was graded as none, mild, moderate or severe. Inter-rater reliability was tested on a subset of scans by two independent and blinded neuroradiologists. Surviving infants underwent the Bayley Scales of Infant and Toddler Development-III (Bayley-III) at 18–24 months. Data were analyzed using univariate and multivariate linear and logistic regression.
Results
Fifty-seven eligible neonates underwent at least one MRI scan in the first 2 weeks of life. Mean postnatal age at scan 1 was 4±2 days in 50/57 (88%) neonates and 48/54 (89%) surviving infants underwent scan 2 at 10±2 days. In 54/57 (95%) survivors, higher MRI injury grades were significantly associated with worse outcomes in the cognitive, motor and language domains of the Bayley-III.
Conclusion
A qualitative MRI injury scoring system weighted for deep nuclear gray matter injury is a significant predictor of neurodevelopmental outcome at 18–24 months in neonates with HIE.
Keywords: Brain, Hypoxic-ischemic encephalopathy, Magnetic resonance imaging, Neonates, Neurodevelopmental outcome, Scoring system
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) is a major worldwide cause of mortality and morbidity occurring in 1 to 6 per 1,000 live births [1, 2]. The introduction of therapeutic hypothermia as a treatment for HIE has decreased mortality and increased disability-free survival in neonates with moderate to severe encephalopathy [3]. However, 40% of survivors demonstrate a spectrum of neurodevelopmental outcome ranging from mild to severe disability, despite treatment with therapeutic hypothermia [3].
Advancements in MRI techniques have improved our ability to evaluate the timing, severity and extent of injury [4, 5]. Integrating the MRI scan into clinical practice facilitates parental counseling on long-term outcomes and enables end-of-life discussions in cases where injury is global and devastating [5–11]. In particular, injuries to deep nuclear gray matter and the posterior limb of the internal capsule are associated with worse neurodevelopmental outcomes [5, 12–15]. Current MRI injury scoring systems published in the literature include the Barkovich, the National Institutes for Child Health and Development (NICHD) and Rutherford systems [10, 16–18]. The latter two systems do not include diffusion-weighted imaging (DWI) and include scans beyond the first 2 weeks of life while the former does not specifically address posterior limb of the internal capsule injury.
To address some of these concerns, we published a clinical MRI scoring system specifically weighted for subcortical injury in the deep nuclear gray matter and posterior limb of the internal capsule. This system incorporates T1-weighted, T2-weighted and DWI sequences to evaluate the timing and extent of injury in HIE using two MRI scans obtained in the time frame recently recommended by a joint task force of the American College of Obstetrics and Gynecology (ACOG) and American Academy of Pediatrics [19, 20]. The scoring system is based on the presence and extent of signal abnormality by region on each sequence and can be applied into clinical practice.
The aim of this study was to validate this MRI scoring system [20] in neonates with moderate to severe neonatal encephalopathy secondary to HIE, using clinical MRI sequences with neurodevelopmental outcomes at 18–24 months of life.
Materials and methods
Neonates with moderate to severe HIE were recruited from the neonatal intensive care unit (NICU) at St. Louis Children’s Hospital as part of a prospective electroencephalography monitoring study from 2007 to 2011. Eligible neonates were ≥35 weeks of gestation with moderate to severe HIE as defined by NICHD criteria [21] who underwent treatment with therapeutic hypothermia. Additional neonates with moderate to severe HIE, admitted between January 2011 and December 2013, who met identical inclusion criteria and had neurodevelopmental testing at 18–24 months were also included. Maternal and neonatal characteristics were obtained from the medical chart. The study protocol was reviewed and approved by the Human Research Protection Office at the Washington University School of Medicine in St. Louis. Informed consent was obtained for all individual participants included in the study.
Imaging protocol
Neonates with moderate to severe HIE underwent at least one MRI scan during hospitalization. By a priori definition, an MRI scan was labeled scan 1 if it occurred in the first 6 days of life and scan 2 if it occurred between 7 and 14 days of life. All neonates were transported to the scanner using institutional neonatal MRI guidelines without sedation [22]. Clinical MRI scans were performed on either a Siemens 1.5-T Avanto or 3.0-T Trio (Siemens Medical, Erlangen, Germany) scanner. The clinical scan sequences on both the1.5- and 3-T scanners were optimized to match resolution and signal-to-noise by an MRI technologist and neuroradiologist. Repetition time (TR) and the number of slices were altered depending on the occipital frontal circumference of the baby to minimize scan time. The custom diffusion sequence consisted of 2×2×2 mm voxels; 9,300 ms TR; 96 ms echo time (TE); 1,710 Hz/Px and 2 b-values 0 and 1,000. Specific differences in sequence parameters between magnets are shown in Table 1.
Table 1.
MRI pulse sequence parameters
| MRI strength | MPRAGE/T1 | T2TSE | DTI |
|---|---|---|---|
| Siemens 1.5-T Avanto |
TR/TE = 1,800 ms/2.99 ms | TR/TE = 3,200 ms/379 ms | TR/TE = 8,100 ms/99ms |
| 1×1×1 mm voxels | 1×1×1 mm voxels | 2×2×3 mm voxels | |
| 15-degree flip angle | |||
| Siemens 3-T Trio |
TR/TE = 1,550 ms/3.05ms | TR/TE = 3,400 ms/132ms | TR/TE = 12,799 ms/99 ms |
| 1×1×1 mm voxels | 1×1×1 mm voxels | 2×2×2 voxels | |
| 15-degree flip angle |
DTI diffusion tensor imaging, MPRAGE magnetization prepared rapid gradient echo imaging, TE echo repetition, TR repetition time, TSE turbo spin echo
A single experienced reader in neonatal neuroimaging blinded to the infant’s clinical course scored the MRI injury (A.M.M., with 14 years of experience) using a previously described scoring method on each scan [20]. If the neonate underwent two scans, both scans were scored and the scan with the higher injury score was used for neurodevelopmental outcome analysis. Interobserver testing was conducted using the single experienced reader (A.M.M.) and two additional independent experienced pediatric neuroradiologists (J.S.S., with 15 years of experience, and R.C.M., with 17 years of experience) blinded to the clinical course scored MRI injury on a subset of scans (n=10). Kappa values were calculated to determine inter-rater reliability for MRI injury grade.
Scoring system (Table 2)
Table 2.
MRI scoring system
| Region | Score |
|---|---|
| Subcortical region | 0 = No signal abnormality |
| • Caudate nucleus | 1 = Signal abnormality in <25% of the region |
| • Globus pallidus/putamen | 2 = Signal abnormality in 25–50% of the region |
| • Thalamus | 3 = Widespread injury involving >50% of the region |
| • Posterior limb of the internal capsule (PLIC) White matter Cortex Cerebellum Brainstem |
On Tl- and T2-weighted imaging, the PLIC is graded from 0 = well myelinated (>50%), 1 = partially myelinated (25–50%), 2 = minimally myelinated (<25%) to 3 = absent myelination. On diffusion-weighted imaging, the PLIC is graded on the area of diffusion restriction from no restriction (0) to extensive restricted diffusion (3). |
| Regional subscore: Summation of independent Tl + T2 + DWI images for the region | |
| MRI injury grade: Summation of the 5 regional subscores | |
| • 0 = No injury | |
| • 1–11 = Mild injury | |
| • 12–32 = Moderate injury | |
| • 33–138 = Severe injury | |
DWI diffusion-weighted imaging
The MRI injury scoring system is an adaptation of a previously published system developed at our institution and is specifically weighted for deep nuclear gray matter and posterior limb of the internal capsule injuries [20]. The five regions assessed qualitatively for injury include (a) the subcortical region made up of the deep nuclear gray matter (i.e. the caudate nucleus, globus pallidus and putamen, and the thalamus) and the posterior limb of the internal capsule; (b) white matter;(c) cortex, (d) cerebellum and (e) brainstem. Each subcortical component was scored independently and added to derive a subcortical region score. Each region was scored based on signal abnormality as either 0 (no injury), 1 (<25% of region), 2 (25–50% of region) or 3 (>50% of region) in each hemisphere on T1-weighted, T2-weighted and DWI culminating in a six-part score per region. The rationale of summating signal abnormalities across the three sequences is that injury seen on multiple sequences is likely more severe than injury seen on only one sequence. Since the brainstem is smaller in comparison to other regions, it was scored from 0 to 2 in all three sequences. A cumulative score was determined by adding up the 5 regional subscores (subcortical+white matter+cortical+cerebellar+brainstem), and classified as no injury (score=0), mild injury (score=1–11), moderate injury (score=12–32) and severe injury (score=33–138). The scoring cutoffs were predetermined in a previous publication [20] and the objective of this project was to validate the scoring system. White matter and cortical regions were presented as a composite in certain analysis to represent the “watershed” region that other MRI injury scoring systems incorporate into their overall score. Examples of MRI regional scores are shown in Fig. 1. The format of the MRI scoring sheet is shown in the Appendix (available for online viewing).
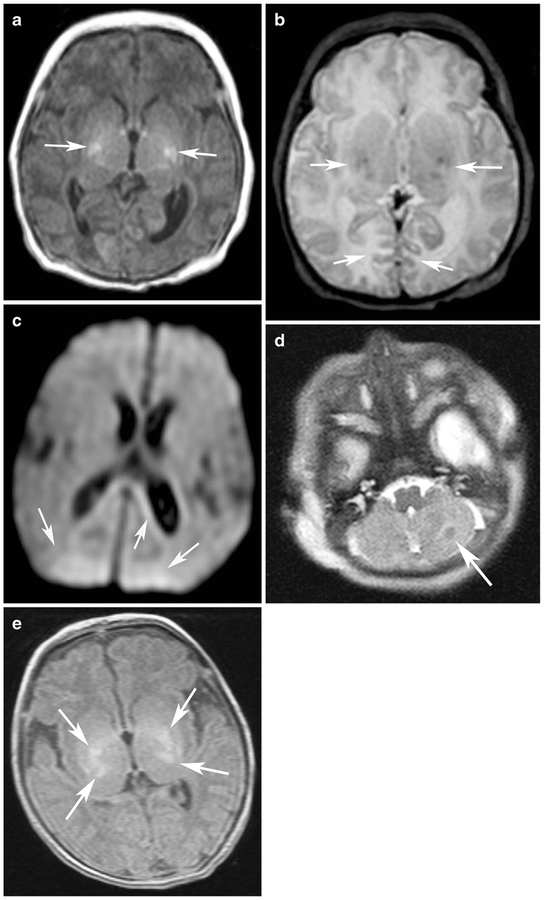
Fig. 1.
Examples of MRI regional scores. a T1-W image score of bilateral globus pallidus/putamen injury (2+2=4) (T1 score=4) (arrows indicate regions of globus pallidus/putamen injury) in a 9-day-old girl (gestational age of 40 weeks). b T2-W image score of bilateral globus pallidus/putamen injury (2+2=4) and bilateral cortical injury (1+1=2) (T2 score=6) (arrows indicate regions of globus pallidus/putamen and cortical injury) in an 11-day-old girl (gestational age 40 weeks)). c Diffusion-weighted MRI image of bilateral restriction in the white matter (1+1=2) (DWI score=2) (arrows indicate regions of white matter injury) in a 5-day-old girl (gestational age of 38 weeks). d T2-W image score of unilateral cerebellar injury (1) (T2 score=2) (arrow indicates region of cerebellar injury) in a 1-day-old girl (gestational age of 36 weeks). e T1-W image score of bilateral global pallidus/putamen (2+2=4) and thalamus injury (2+2=4) (T1 score=8) (arrows indicate regions of globus pallidus/putamen and thalamic injury) in a 10-day-old boy (gestational age of 40 weeks)
Neurodevelopment follow-up
Survivors underwent the Bayley Scales of Infant and Toddler Development-III (Bayley-III) between 18 and 24 months administered by a blinded psychologist. The primary outcome included cognitive, language and motor composite scores on the Bayley-III. The secondary outcome was dichotomized as “good” (Bayley-III score of ≥85 in all three domains) or “adverse” (death or a Bayley-III score of <85 in any domain) [23].
Statistical analysis
Statistics were estimated for demographic data as means and standard deviations [SD]. Comparisons of clinical variables were made with a Fisher exact test and two-tailed t-test. The relationship between MRI cumulative scores, MRI injury grade and Bayley-III neurodevelopmental scores were compared using univariate linear regression analysis. Binary logistic regression was used to calculate the likelihood of adverse outcome (defined as death or Bayley-III <85 in any domain) given a particular MRI injury grade. Analysis of variance (ANOVA) was conducted to compare the differences in mean MRI scores among three neurodevelopment impairment severity categories for each individual Bayley-III domain. Multivariate linear regression was used to evaluate the neurodevelopmental consequences of injury in each region while controlling for injury elsewhere. Two-sided P-value ≤0.05 was defined to be significant. A receiver operator characteristic (ROC) curve for the MRI injury cumulative score with the dichotomized outcome of “good” and “adverse” was produced to determine a cutoff MRI cumulative score. Data was analyzed using IBM SPSS Statistics Version 21 (IBM Corporation, Chicago, IL, USA).
Results
Between 2007 and 2013, 57 neonates with moderate to severe HIE, treated with therapeutic hypothermia, underwent at least one MRI scan in the first 2 weeks of life. Ninety-five percent of the infants (54/57; 3 infants died) returned for neurodevelopmental assessment between 18 and 24 months. Scoring a subset of MRI scans on 10 infants, the 3 MRI readers had substantial agreement on MRI injury grade, producing a kappa value of 0.81. By testing the standardized residuals, the Bayley-III scores are normally distributed. Demographic and clinical data for the 57 neonates included in the study are shown in Table 3.
Table 3.
Clinical characteristics of the cohort
| Sample (n=57) |
|
|---|---|
| Gestational age at birth, mean ± SD, weeks | 38.5±1.6 |
| Birth weight, mean ± SD, grams | 3166±688 |
| Race, n (%) | |
| • Caucasian | 35 (61) |
| • African-American | 18(32) |
| • Hispanic | 3(5) |
| • Asian | 1(2) |
| Male sex, n (%) | 28 (49) |
| 5 min APGAR score, median | 4 |
| 10 min APGAR score, median | 5 |
| Cord blood/First arterial/venous pH, mean ± SD | 7.05±0.19 |
| Cord blood/First arterial/venous Base deficit, mean ± SD | −14.18±6.87 |
| Severity of HIE, n (%) | |
| • Moderate | 46(81) |
| • Severe | 11(19) |
| Delivery location, n (%) | |
| • In born | 28 (49) |
| • Out born | 29(51) |
| Mode of delivery, n (%) | |
| • Vaginal | 18(32) |
| • Cesarean section | 39 (68) |
| Infant deaths, n (%) | 3(5) |
| MRI scans per signal strength, n (%)a | |
| • 1.5 T | 51 (51%) |
| •3.0 T | 49 (49%) |
| MRI injury grade, n (%) | |
| • No injury | 21 (37) |
| • Mild injury | 19 (33) |
| • Moderate injury | 11 (19) |
| • Severe injury | 6(11) |
Total number of scans performed equals 100 on the 57 infants included in the cohort
HIE hypoxic-ischemic encephalopathy, SD standard deviation
MRI injury score and neurodevelopmental outcome
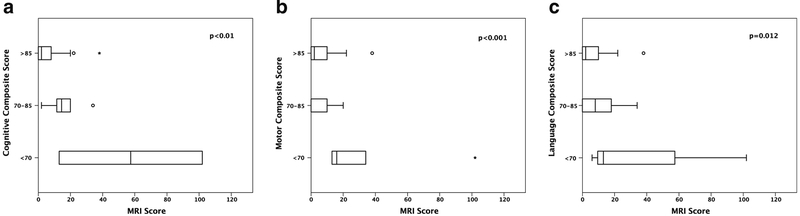
Univariate analysis demonstrated a significant association between higher MRI injury scores and lower Bayley-III scores in the cognitive, motor and language domains. Individual R2 and P-values are shown in Table 3. When grouping the individual Bayley-III domains composite scores by severity of neurodevelopmental impairment (greater than 85 [less than 1 SD], between 70 and 85 [between 1 and 2 SD] and less than 70 [more than 2 SD away from the average]), there is a statistically significant difference in the mean MRI scores among the 3 neurodevelopment impairment severity categories for each individual Bayley-III domain (Cognitive: F=5.9, P<0.01, Motor: F=4.1, P<0.01, Language: F=2.5, P<0.01) (Fig. 2).
Fig. 2.
MRI injury score and severity of developmental delay by domain. a Box and whisker plot compares MRI injury score with Bayley-III cognitive domain separated into categories by severity of neurodevelopmental impairment. X-axis displays MRI injury score. Y-axis displays the individual Bayley-III domain neurodevelopmental impairment categories. b Box and whisker plot compares MRI injury score with Bayley-III motor domain separated into categories by severity of neurodevelopmental impairment. X-axis displays MRI injury score. Y-axis displays the individual Bayley-III domain neurodevelopmental impairment categories. c Box and whisker plot compares MRI injury score with Bayley-III language domain separated into categories by severity of neurodevelopmental impairment. X-axis displays MRI injury score. Y-axis displays the individual Bayley-III domain neurodevelopmental impairment categories
MRI injury score and adverse outcome
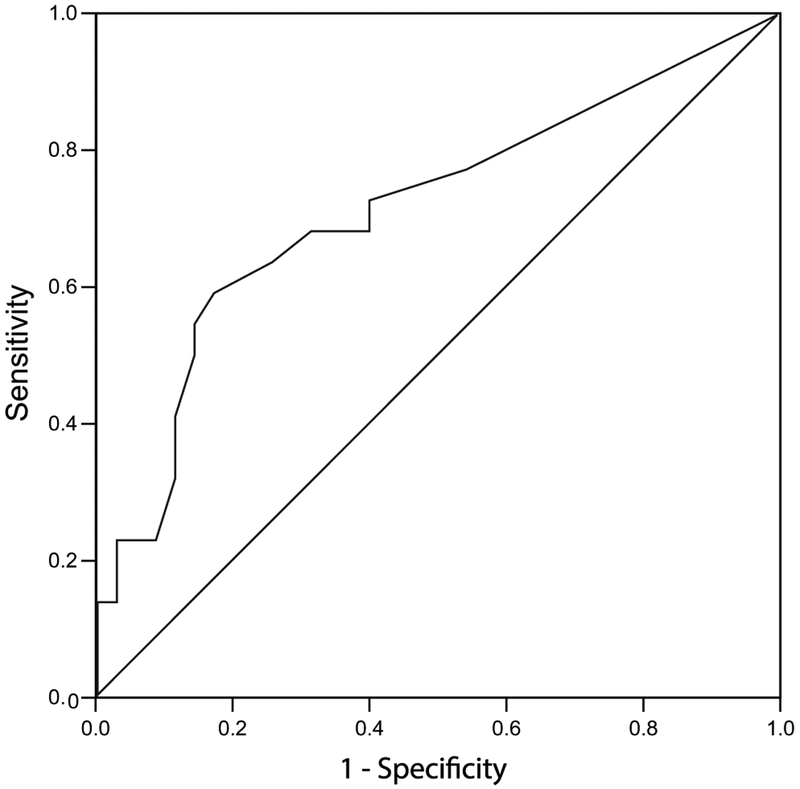
Using the dichotomized outcome of “good” and “adverse,” an ROC curve for the MRI injury cumulative score was produced (Fig. 3). The area under the curve was 0.72 with a 95% confidence interval [CI] of 0.57–0.86 (P=0.007). The point on the curve with the highest sensitivity and specificity occurred between a MRI cumulative score of 10.5 and 11.5. This corresponds with the cutoff value between mild and moderate grade of injury.
Fig. 3.
Receiver operator characteristic curve for detecting an adverse outcome using MRI injury score. X-axis displays 1-Specificity. Y-axis displays sensitivity
MRI injury on scan 1 versus scan 2
Forty-one of the 47 surviving (87%) neonates with scan 1 had scan 2 at a mean postnatal age of 10±2 days. Scan 2 demonstrated the worst grade of injury in 38/41 (92%) neonates. Thirty-two of 41 (78%) neonates showed no change in MRI injury grade between scans 1 and 2. Three of 9 (33%) showed improvement in MRI injury grade whereas MRI injury grade worsened in 6/9 (66%).
MRI injury grade and neurodevelopmental outcome
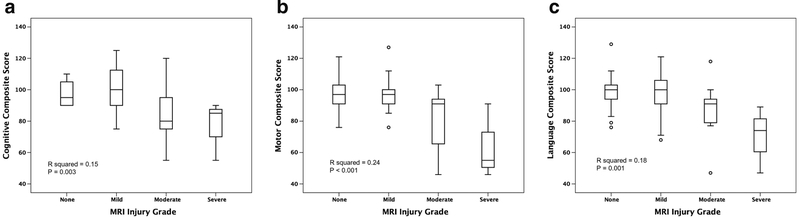
Univariate analysis demonstrated a significant association between higher MRI injury grades and lower Bayley-III scores in the cognitive, motor and language domains (Fig. 4). Individual R2 and P-values are shown in Table 4. Binary logistic regression demonstrated an increased likelihood of an adverse outcome as the MRI injury grade increases (odds ratio [OR]: 2.2, 95% CI: 1.1–4.3, P=0.02). Of the 54 neonates who survived to neurodevelopmental follow-up, 35 had a “good outcome,” 12 developed cerebral palsy and 1 had post-neonatal epilepsy. Among the 22 infants with an adverse outcome, 17 had an MRI injury; of the 35 infants with good outcome, 16 had no injury on MRI (sensitivity 0.77, specificity 0.46, positive predictive value 0.47 and negative predictive value 0.76).
Fig. 4.
MRI injury grade and neurodevelopmental outcomes by domain. a Box and whisker plot compares MRI injury grade with Bayley-III cognitive domain. X-axis displays MRI injury grade. Y-axis displays the individual Bayley-III domain. b Box and whisker plot compares MRI injury grade with Bayley-III motor domain. X-axis displays MRI injury grade. Y-axis displays the individual Bayley-III domain. c Box and whisker plot compares MRI injury grade with Bayley-III language domain. X-axis displays MRI injury grade. Y-axis displays the individual Bayley-III domain
Table 4.
Regression analysis of MRI injury score and MRI injury grade on Bayley-III composite scores
| Measure | Cognitive | Motor | Language | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | R2 | P-value | Beta | R2 | P-value | Beta | R2 | P-value | |
| MRI injury score (w=54) | −0.46 | 0.21 | <0.001* | −0.53 | 0.28 | <0.001* | −0.50 | 0.25 | 0.012* |
| MRI injury grade (n=54) | −0.39 | 0.15 | 0.003* | −0.49 | 0.24 | <0.001* | −0.42 | 0.18 | 0.001* |
All univariate regression models significant at P<0.05. All beta values are standardized
Denotes significance at P<0.05
Regional MRI injury and neurodevelopmental outcome
Using multivariate linear regression analysis, higher (white matter and cortical) injury scores were independently associated with lower scores in the cognitive domain when adjusted for other areas of injury. Subcortical (deep nuclear gray matter and posterior limb of the internal capsule) and (white matter and cortical) regional injury were independently associated with lower motor domain scores and subcortical injury was independently associated with lower scores in the language domain. The complete model output is shown in Table 5. Nine newborns demonstrated brainstem injury and six newborns had cerebellar injury, primarily associated with more widespread supratentorial injury.
Table 5.
Regression analysis of regional injury on Bayley-III composite scores
| Measure | Cognitive | Motor | Language | |||
|---|---|---|---|---|---|---|
| Beta | P-value | Beta | P-value | Beta | P-value | |
| Regional injury (n=54) | ||||||
| Subcortical | −0.03 | 0.89 | −0.50 | 0.002* | −0.54 | 0.001* |
| White matter/cortical | −0.52 | 0.001* | −0.31 | 0.04* | −0.28 | 0.07 |
| Brainstem | −0.06 | 0.74 | 0.21 | 0.22 | 0.27 | 0.11 |
| Cerebellum | −0.04 | 0.75 | −0.03 | 0.82 | −0.06 | 0.64 |
The multivariate regression model is significant at P<0.05. All beta values are standardized
Denotes significance at P<0.05
Discussion
In this single-center study, we validated a MRI scoring system weighted for subcortical (deep nuclear gray matter and posterior limb of the internal capsule) injury that includes clinically obtained T1-weighted, T2-weighted and DWI sequences with neurodevelopmental outcome between 18 and 24 months in neonates with moderate to severe HIE treated with therapeutic hypothermia. Scans were performed within the time frames recommended by the ACOG task force on neonatal encephalopathy [19]. Across the cohort, increasing MRI injury scores and grades were significantly associated with lower performance scores across all three domains of the Bayley-III. When categorizing each Bayley-III domain into severity of neurodevelopmental disability, the relationship persisted. The three readers had substantial agreement on the MRI injury grade in all cases used in the tested subset (n=10), producing an inter-rater reliability of 81% in determining whether there was no, mild, moderate or severe injury.
Two main patterns of injury in neonatal HIE are well documented in the literature: deep nuclear gray matter and water-shed injury (involving intervascular boundary-zone white matter, plus cortical gray matter) [5, 12, 16, 24, 25]. The association of the predominant pattern of injury on MRI is a strong predictor of neurodevelopmental outcome [5]. In 2002, Barnett et al. [26] concluded that moderate or severe basal ganglia lesions were the best predictor of cerebral palsy. Mulkey et al. [25] described the effect of injury in deep nuclear gray matter on long-term neurodevelopmental outcome and cerebral palsy in a cohort of neonates with HIE enrolled as part of the CoolCap Trial. Due to the prior literature documenting the effect of deep nuclear gray matter and posterior limb of the internal capsule injury on neurodevelopmental outcome, we developed our scoring system to be weighted for such injury.
The watershed pattern of injury is often considered milder and with minimal effect on neurodevelopmental outcome at 18 months [27]. However, after adjusting for other regions of injury, multivariate analysis of our cohort showed that water-shed injury is significantly associated with worse outcomes in the cognitive domain at 18–24 months. These results add to the recent concerns of cognitive delay identified at 30 months of age in neonates with watershed injury [5, 28]. These results highlight the importance of close follow-up as developmental interventions may need to be initiated sooner in infants with a watershed-type injury.
DWI is a valuable MRI sequence with the ability to accurately detect injury as early as 24–72 h of life, and when combined with T1-W, T2-W and DWI sequences in the first 2- to 5-day window provides an indication of timing of injury [19, 29–31]. The Barkovich system initially used only T1-W and T2-W sequences. This was subsequently modified to include DWI in the first 2 weeks of life. The system, however, does not explicitly include the posterior limb of the internal capsule in its scoring system, a region with excellent predictive value of injury [14, 15, 32]. The Rutherford and NICHD MRI scoring systems do not incorporate diffusion sequences and, being based on multicenter hypothermia trial cohorts, have heterogeneous timing of the MRI scans after birth with the former system consisting of a range of 2–30 days [10] and the latter consisting of a mean ± SD of 15±12 days [16].
The executive task force on neonatal HIE from the ACOG published an executive summary in 2014 outlining recommendations for neuroimaging in the setting of neonatal encephalopathy. They recommend two MRI scans when feasible; one obtained early in the neonatal course (24–96 h), which is useful in delineating the timing of perinatal injury, and the second between 7 and 21 days of life, which is important to determine the extent of injury with an ideal goal of obtaining a second MRI scan on day of life 10 [19]. A major strength of our study is that the timing of MRI scans and sequences fall within the recommendation by this task force. In our study, the average postnatal age ± SD at scan 1 was 4±3 days of life with a large proportion (72%) having a second scan at 10±2 days of life.
Another strength of our study is the validation of the MRI scoring system with the gold standard test for neurodevelopmental outcome, i.e. the Bayley-III at an optimal age of 18–24 months. Validation of our scoring system against an established neurodevelopmental assessment tool allows for earlier identification of the potential functional impairment and can provide physicians with the information to guide management, specifically developmental therapy through intervention programs [9, 29, 33].
When including the three neonates who died prior to neurodevelopmental testing, the OR of developing an adverse outcome is 2.2 times higher for each increase in MRI injury grade. Not only does the MRI scoring system predict neurodevelopmental outcome based on a MRI injury grade, it provides information on the risk of developing an adverse outcome, a framework that clinicians and parents can understand.
A limitation of our study is the heterogeneity of magnet field strength, with some infants undergoing scans in a 1.5-T scanner and others in a 3-T scanner. However, this situation mimics what happens in clinical practice across centers and the quality of basic scan sequences, such as T1-W, T2-W and DWI, is sufficient on all modern scanners for qualitative interpretation needed to score the images. The scoring system retained validity despite the varying magnet strength. Another limitation of this scoring system is that it can only be used in the first 2 weeks of life as the majority of diffusion restriction is resolved beyond this time window [20]. Lastly, the R2 values are on the lower end, which could be secondary to the low cohort size in the severe group (babies who died were not included since they did not have a Bayley-III score). Nevertheless, we think that the R2 values, even though low, demonstrate a valuable association especially since the P-values remain significant.
Conclusion
A qualitative MRI injury scoring system using clinical MRI sequences – weighted for deep nuclear gray matter and posterior limb of the internal capsule injury and conducted in the time frame suggested by the ACOG summary statement on HIE – is a significant predictor of neurodevelopmental outcome at 18–24 months in neonates with HIE treated with therapeutic hypothermia.
Supplementary Material
Acknowledgements
The authors wish to thank Anthony Barton (no conflicts of interest) for his efforts in coordinating the project and the infants and families for their generous assistance and dedication. In addition, we are grateful for the support from the Thrasher Foundation and the Washington University KL2 program.
Funding was provided by the Thrasher Foundation, the Washington University Institute of Clinical and Translational Sciences KL2 Training Program (NIH/NCATS KL2 TR000450–08), and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University.
Footnotes
Compliance with ethical standards
Conflicts of interest None
References
- 1.Chau V, Poskitt KJ, Dunham CP et al. (2014) Magnetic resonance imaging in the encephalopathic term newborn. Curr Pediatr Rev 10: 28–36 [DOI] [PubMed] [Google Scholar]
- 2.Volpe J (2001) Hypoxic-ischemic encephalopathy: clinical aspects in neurology of the newborn. WBS Company, Philadelphia [Google Scholar]
- 3.Jacobs SE, Berg M, Hunt R et al. (2013) Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 1: CD003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan F, Rutherford M, Groenendaal F et al. (2003) Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 361:736–742 [DOI] [PubMed] [Google Scholar]
- 5.Miller SP, Ramaswamy V, Michelson D et al. (2005) Patterns of brain injury in term neonatal encephalopathy. J Pediatr 146:453–460 [DOI] [PubMed] [Google Scholar]
- 6.Thayyil S, Chandrasekaran M, Taylor A et al. (2010) Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 125:e382–e395 [DOI] [PubMed] [Google Scholar]
- 7.Skranes JH, Cowan FM, Stiris T et al. (2015) Brain imaging in cooled encephalopatic neonates does not differ between four and 11 days after birth. Acta Paediatr 104:752–758 [DOI] [PubMed] [Google Scholar]
- 8.Bell E, Rasmussen LA, Mazer B et al. (2015) Magnetic resonance imaging (MRI) and prognostication in neonatal hypoxic-ischemic injury: a vignette-based study of Canadian specialty physicians. J Child Neurol 30:174–181 [DOI] [PubMed] [Google Scholar]
- 9.Massaro AN, Kadom N, Chang T et al. (2010) Quantitative analysis of magnetic resonance images and neurological outcome in encephalopathic neonates treated with whole-body hypothermia. J Perinatol 30:596–603 [DOI] [PubMed] [Google Scholar]
- 10.Rutherford M, Ramenghi LA, Edwards AD et al. (2010) Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 9:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wintermark P, Hansen A, Soul J et al. (2011) Early versus late MRI in asphyxiated newborns treated with hypothermia. Arch Dis Child Fetal Neonatal Ed 96:F36–F44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutherford M, Srinivasan L, Dyet L et al. (2006) Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol 36:582–592 [DOI] [PubMed] [Google Scholar]
- 13.Mercuri E, Rutherford M, Barnett A et al. (2002) MRI lesions and infants with neonatal encephalopathy. Is the Apgar score predictive? Neuropediatrics 33:150–156 [DOI] [PubMed] [Google Scholar]
- 14.Rutherford MA, Pennock JM, Counsell SJ et al. (1998) Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 102:323–328 [DOI] [PubMed] [Google Scholar]
- 15.Hunt RW, Neil JJ, Coleman LT et al. (2004) Apparent diffusion coefficient in the posterior limb of the internal capsule predicts outcome after perinatal asphyxia. Pediatrics 114:999–1003 [DOI] [PubMed] [Google Scholar]
- 16.Shankaran S, Barnes PD, Hintz SR et al. (2012) Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 97:F398–F404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkovich AJ, Hajnal BL, Vigneron D et al. (1998) Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 19:143–149 [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifacio SL, Glass HC, Vanderpluym J et al. (2011) Perinatal events and early magnetic resonance imaging in therapeutic hypothermia. J Pediatr 158:360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Alton M (2014) Executive summary: neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ task force on neonatal encephalopathy. Obstet Gynecol 123:896–901 [DOI] [PubMed] [Google Scholar]
- 20.Bednarek N, Mathur A, Inder T et al. (2012) Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 78:1420–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankaran S, Laptook AR, Ehrenkranz RA et al. (2005) Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 353:1574–1584 [DOI] [PubMed] [Google Scholar]
- 22.Mathur AM, Neil JJ, McKinstry RC et al. (2008) Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol 38:260–264 [DOI] [PubMed] [Google Scholar]
- 23.Johnson S, Moore T, Marlow N (2014) Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res 75:670–674 [DOI] [PubMed] [Google Scholar]
- 24.Barkovich AJ (2006) MR imaging of the neonatal brain. Neuroimaging Clin N am 16:117–135, viii–ix [DOI] [PubMed] [Google Scholar]
- 25.Mulkey SB, Yap VL, Swearingen CJ et al. (2012) Quantitative cranial magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol 47:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnett A, Mercuri E, Rutherford M et al. (2002) Neurological and perceptual-motor outcome at 5–6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics 33:242–248 [DOI] [PubMed] [Google Scholar]
- 27.de Vries LS, Jongmans MJ (2010) Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 95:F220–F224 [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez FF, Miller SP (2006) Does perinatal asphyxia impair cognitive function without cerebral palsy? Arch Dis Child Fetal Neonatal Ed 91:F454–F459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gano D, Chau V, Poskitt KJ et al. (2013) Evolution of pattern of injury and quantitative MRI on days 1 and 3 in term newborns with hypoxic-ischemic encephalopathy. Pediatr Res 74:82–87 [DOI] [PubMed] [Google Scholar]
- 30.McKinstry RC, Miller JH, Snyder AZ et al. (2002) A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 59:824–833 [DOI] [PubMed] [Google Scholar]
- 31.Azzopardi D, Edwards AD (2010) Magnetic resonance biomarkers of neuroprotective effects in infants with hypoxic ischemic encephalopathy. Semin Fetal Neonatal Med 15:261–269 [DOI] [PubMed] [Google Scholar]
- 32.de Vries LS, Groenendaal F (2010) Patterns of neonatal hypoxicischaemic brain injury. Neuroradiology 52:555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalak LF, DuPont TL, Sanchez PJ et al. (2014) Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. J Perinatol 34:629–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.