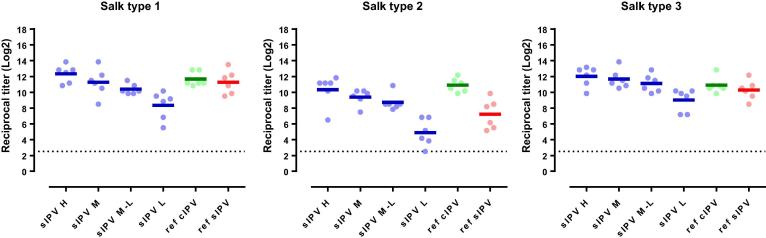
Fig. 4.
Non-inferiority of PER.C6®-based sIPV compared to Sabin IPV (sIPV) and Salk IPV (cIPV) commercial reference vaccines based on Sabin virus neutralizing antibody titers after three immunizations (at week 9). Sera from cynomolgus monkeys, immunized 3 times with one of the four dose formulations of PER.C6®-based sIPV or commercial cIPV or sIPV, were analyzed with Sabin viral neutralizing assay to determine Sabin virus neutralizing antibody titers. The four different D-antigen unit dose formulations were high dose formulation (20-30-100 DU), mid dose (10-15-50 DU), mid low dose (5-7.5-25 DU) and low dose (2.5-3.75-12.5 DU). To assess non-inferiority between the different vaccines, an area under the curve analysis at week 9 was performed. A vaccine formulation is regarded non-inferior compared to the reference vaccine if the lower limit of the 95% confidence interval (CI) of the difference in log10 titer is above −0.6 difference in log10 titer (indicated by the vertical dotted line).