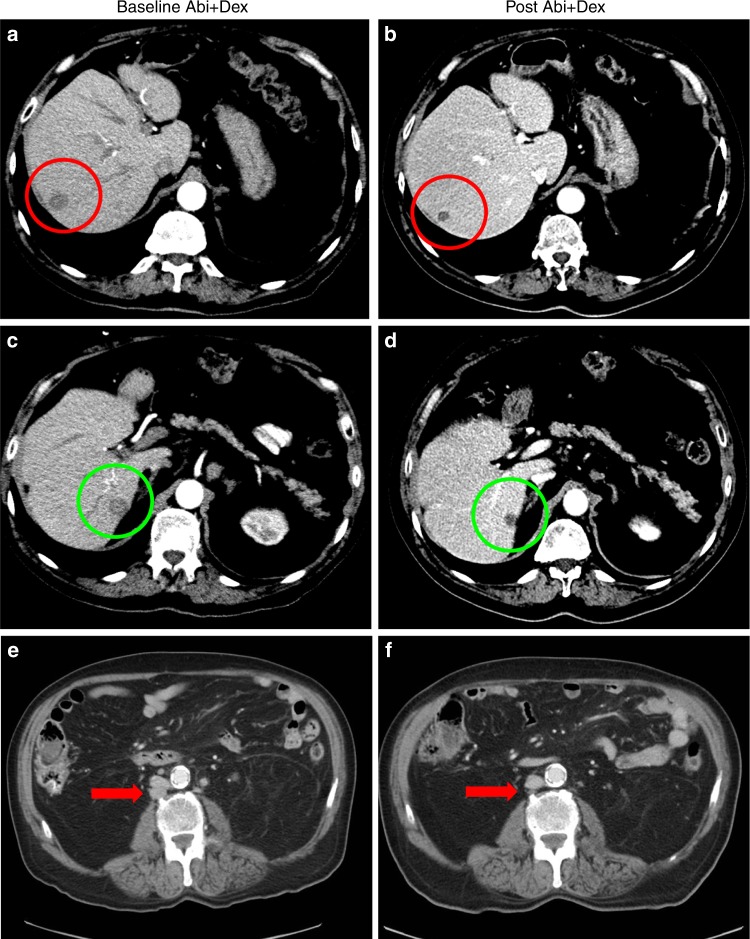
Fig. 3.
a–d illustrate the response of Patient 004, who had previously experienced progression on a luteinizing hormone-releasing hormone agonist (LHRHa) and bicalutamide. After 16 weeks on AA+P he presented with confirmed biochemical and radiological progression of liver metastases (30% increase of target lesions) a and c. Then, patient was switched from concomitant prednisone 5 mg bid to dexamethasone 0.5 mg od. After 12 weeks on AA+D, PSA decreased to a nadir of 0.61 ng/mL (88% decline), and b and d the red and green oval, respectively, show a partial response of his target liver metastases. Patient 004 continued on AA+D until clinical and radiographic progression occurred 13.6 months after the switch. e, f show the response of patient 022. This patient had previously been treated with LHRHa, bicalutamide, ketoconazole/hydrocortisone, and docetaxel/prednisone. Patient responded to AA+P. After 14.8 and 31.3 months he experienced biochemical and radiological progression, respectively. e. Patient 022 was then switched to dexamtehasone and reached a biochemical response ( > 90%) and a significant radiological response of target nodal disease f, red arrow) still ongoing after 19.2 months on the study at the time of data cut–off. Patients 004 and 022 did not presented AR amplification or detectable mutations in ctDNA at time of switch