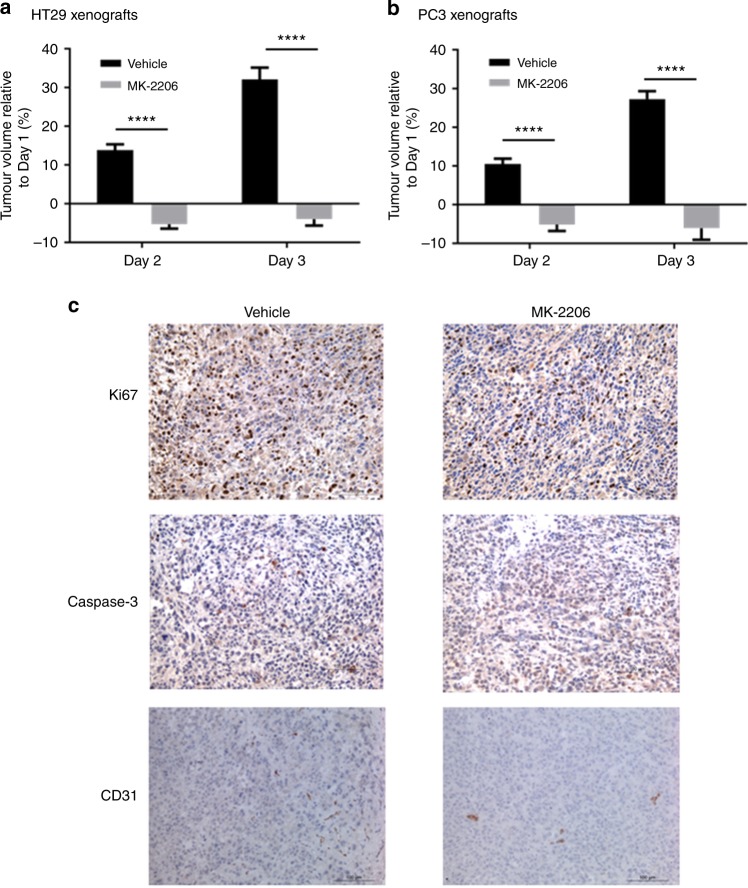
Fig. 2.
Tumour volume and histological changes in subcutaneous tumours following MK-2206 treatment. Percentage change in HT29 (a) and subcutaneous PC3 (b) tumour volumes (relative to Day 1) following 2 doses (Day 1 and 3) of 120 mg/kg of MK-2206 on alternate days via p.o. (n = 10) or vehicle alone (10% DMSO in saline), minimum n = 10. Data are expressed as mean ± SEM, ****P < 0.0001. c Immunohistochemistry of Ki67, caspase-3 and CD31 expressions (brown staining) in vehicle-treated control (left column) and MK-2206 treated (right column) subcutaneous PC3 xenografts (right column). Magnification 200×