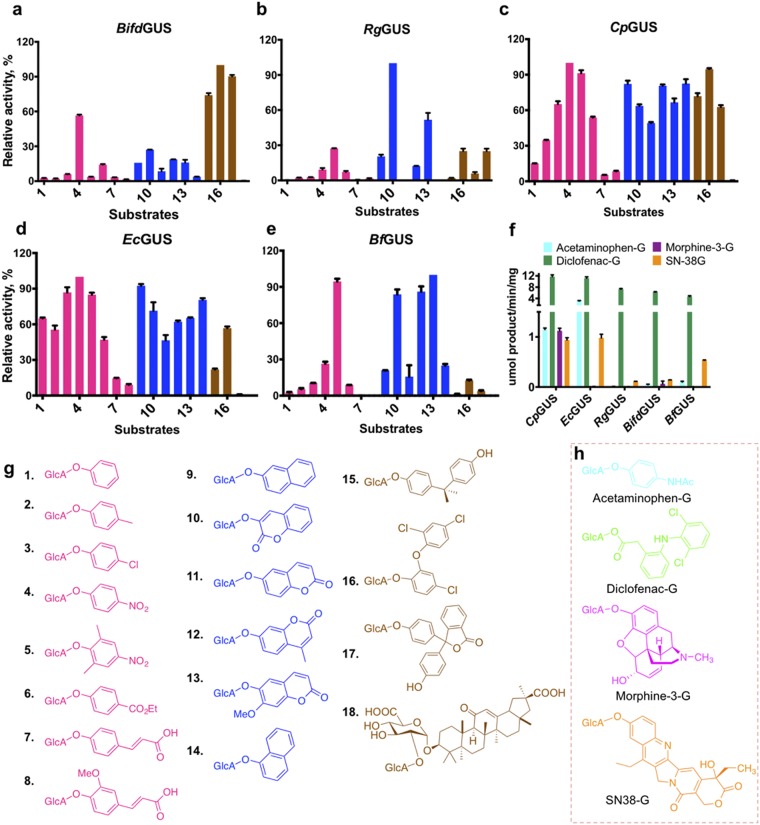
Figure 2.
The five tested gut bacterial GUSs have distinct substrate preferences. Substrate preferences of the five gut bacterial GUSs and molecular structures of the glucuronides are shown. Relative activities of (a) BifdGUS, (b) RgGUS, (c) CpGUS, (d) EcGUS, and (e) BfGUS toward substrates 1–18. The structures of the substrates are shown in (g) and were categorized into three groups according to the type of aglycone as mono-aromatic (pink), fused aromatic (blue), and bulky aglycones (brown). Panel f compares the specific activities of the GUSs for five drug glucuronides with their structures are shown in panel h. Relative activities of the GUSs were calculated as specific activity (μmol/min/mg) and were determined as follows. Enzymes (10 ng of CpGUS and EcGUS, and 50 ng of RgGUS, BfGUS, and BifdGUS) were incubated with 250 μM substrates in 50 mM sodium citrate, 150 mM NaCl at pH 6.0. The highest activities for a given substrate was set as 100%. For CpGUS, EcGUS, RgGUS, BfGUS, and BifdGUS, the greatest activities were for 4, 10, 15, and 16, respectively. (g) Molecular structures of xenobiotic glucuronides used in this study are for: 1, phenoxy; 2, p-methylphenoxy; 3, p-chlorophenoxy; 4, p-nitrophenoxy; 5, 2,6-dimethyl-4-nitrophenoxy; 6, 4-(ethoxycarbonyl)phenoxy; 7, 4-((E)-2-carboxyvinyl)phenoxy; 8, 4-((E)-2-carboxyvinyl)-2-methoxyphenoxy; 9, naphthalen-2-yloxy; 10, 2-oxo-2H-chromen-3-yloxy; 11, 2-oxo-2H-chromen-6-yloxy; 12, 4-methylumbelliferyloxy; 13, 7-methoxy-2-oxo-2H-chromen-6-yloxy; 14, naphthalen-1-yloxy, 15, bisphenyl-A; 16, triclosan; 17, phenolphthalein; and 18, glycyrrhizin. (h) Molecular structures of drug glucuronides.