Figure 4.
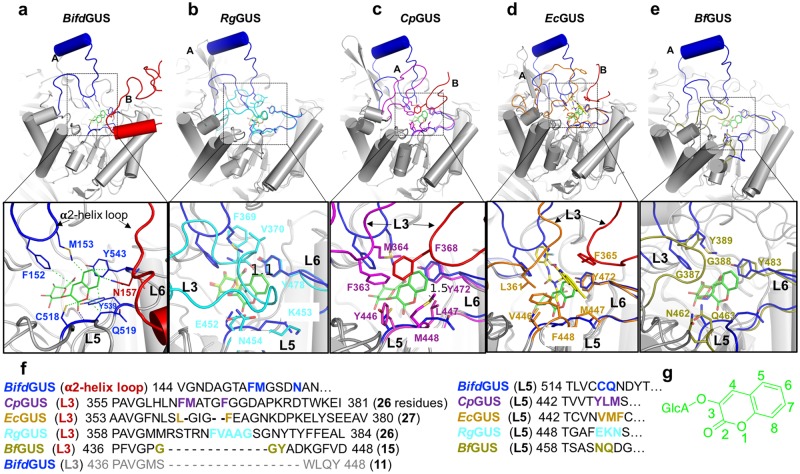
Loop 3 (or the α2-helix loop) and loop 5 are important for the substrate preferences of the GUSs. Comparison of the ABSs of the five bacterial GUSs. The structures of RgGUS/UIFG (b), 4JKM (CpGUS-apo) (c), 3LPG (the EcGUS/N-substituted-thiourea derivative) (d), and BfGUS/UIFG (e) were superimposed on the structure of BifdGUSE479A/10 (a). The substrate-binding sites of GUSs are located at the center of the TIM barrel domains. Panels (a–e) display the ABSs that are formed by the conserved loop 6 (L6) and variable loop 5 (L5) and loop 3 (L3) or α2-helix loop. The α2-helix loop in BifdGUS occupies a position similar to that of loop 3 in the other GUSs. (f) Sequences of loop 3 (or the α2-helix loop) and loop 5 in the five bacterial GUSs. Residues interacting with the aglycone of 10 are shown as blue, cyan, magenta, yellow, and olive sticks in panels a–e, respectively. (g) Molecular structure of 10.