Figure 2.
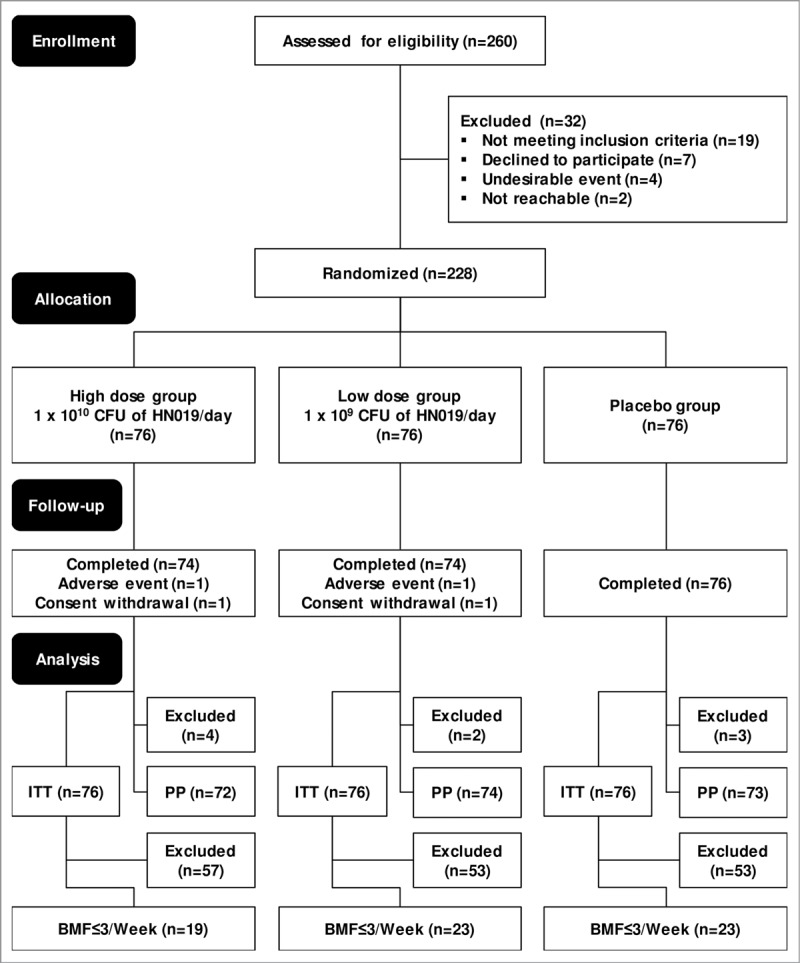
CONSORT diagram of the 28-day, 3-arm parallel-group (allocation ratio 1:1:1), double-blind, randomized, placebo-controlled, and monocenter study, preceded by a 2-week run-in period. BMF ≤ 3/week, participants from the ITT population who had fewer than or equal to 3 bowel movements per week during the entire 2-week run-in period; CONSORT, Consolidated Standards of Reporting Trials; ITT, intention-to-treat population—all participants randomized at the second visit who consumed at least 1 dose of the study product; PP, per-protocol population—participants who attended the end-of-study visit and received at least 80% of the assigned study product and consumed 100% of radio-opaque markers during the intervention on time.
