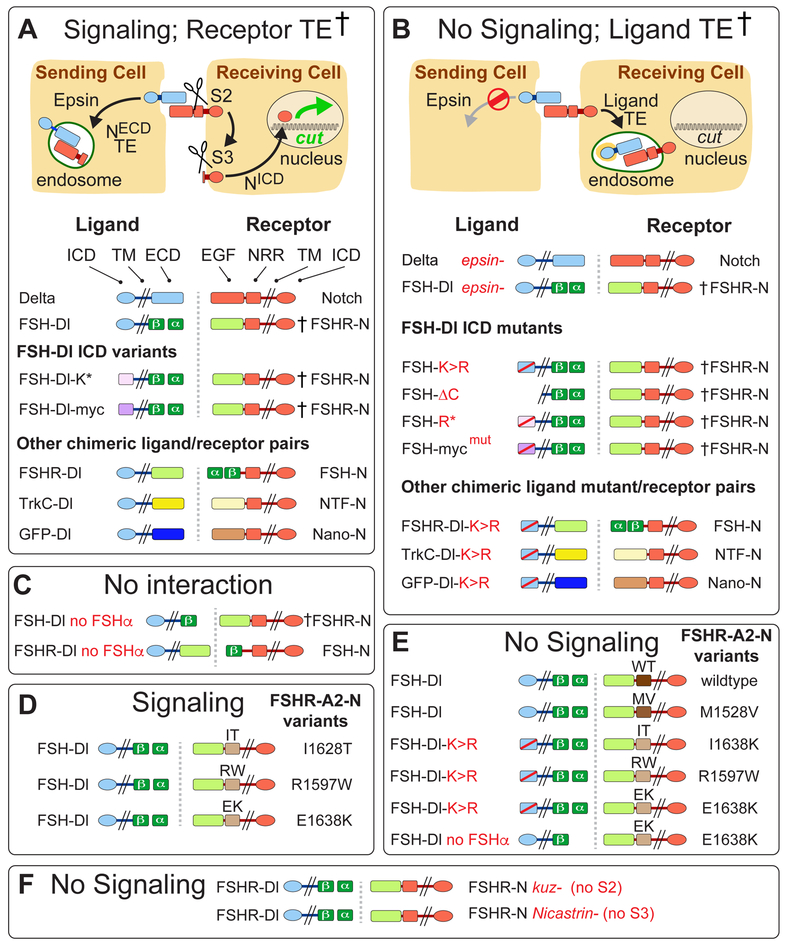
Figure 1. Signaling and endocytic fates of chimeric DSL/Notch pairs.
A) Productive ligand/receptor pairs. Binding of Dl to Notch induces S2 cleavage of the Negative Regulatory Region (NRR), S3 cleavage of the transmembrane (TM) domain, nuclear access of the intracellular domain (ICD) and activation of target genes (e.g., cut); the shed ectodomain is transendocytosed (TE) into the signal sending cell († = ectodomain TE confirmed by experiment).
FSH-Dl/FSHR-N.
The Dl extracellular domain (ECD) was replaced by the β subunit of Follicle Stimulating Hormone (FSHβ) and FSHα was expressed to reconstitute the composite FSH ligand (FSH). Reciprocally, the ligand-binding (EGF) portion of the Notch ectodomain was replaced by the FSH receptor ectodomain (FSHR; see STAR Methods and Figure S1 for composition of all proteins).
FSH-Dl ICD variants.
The Dl ICD was replaced by either (i) an unrelated 38 aa peptide bearing two K’s that target ligand to the Epsin pathway (FSH-Dl-K*; Wang and Struhl, 2004), or (ii) a Myc epitope that includes a LI dipeptide that comprises a Clathrin internalization signal that bypasses the requirement for Epsin (FSH-Dl-myc).
Other ligand/receptor pairs.
Alternative ligand/receptor pairs were generated by swapping the FSH and FSHR domains (FSHR-Dl/FSH-N) or replacing these domains with the Tropomyosin receptor kinase C and Neurotrophin-3 ectodomains (TrkC-Dl/NTF-N), or with GFP and an anti-GFP nanobody (GFP-Dl/Nano-N).
B) Non-productive ligand/receptor pairs. Preventing ligand entry into the Epsin/Clathrin pathway (Ø) by removing Epsin (epsin¯) or by altering the ligand ICD blocks S2 cleavage and results in transendocytosis of ligand into the signal-receiving cell (TE; † = transendocytosis of the ligand ectodomain confirmed by experiment).
FSH-Dl and other chimeric ligand ICD mutants
FSH-Dl, FSH-Dl-K* and FSH-Dl-myc were blocked from entering the Epsin/Clathrin pathway by mutating the cytosolic K’s to R’s (FSH-Dl-K>R, FSH-Dl-R*), or the LI internalization signal to AI (FSH-Dl-mycmut).
C-F) FSH-Dl/FSHR-N signaling and TE require FSHα to reconstitute the functional FSHαβ heterodimer (C), and Kuz and Net to execute the S2 and S3 cleavages (F). FSH-Dl, and not FSH-Dl-K>R, can activate FSHR-A2-N chimeric receptors if they carry any of three disease-associated A2 variants (D,E). FSH-Dl does not activate receptors carrying A2 domains that are cleaved less readily in response to mechanical tension (wildtype, WT, and MV1528; D).