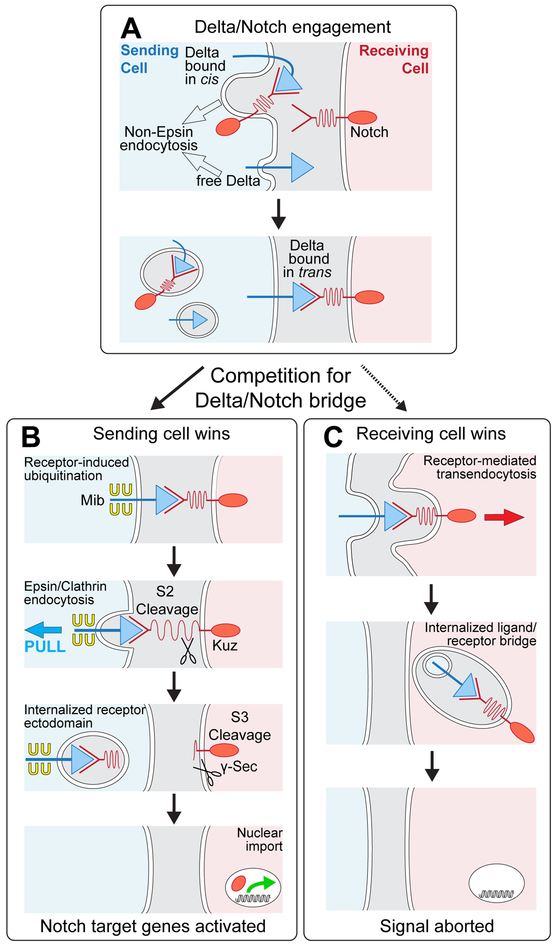
Figure 7. Dl/Notch signaling and competition between sending and receiving cells for the ligand/receptor bridge.
A) Prior to engagement with Notch in trans, Dl exists in two forms: free, and sequestered in cis with Notch; both forms can be internalized via non-Epsin routes. Binding to Notch in trans induces a race between ubiquitination of Dl in the sending cell (B) and uptake of Dl into the receiving cell (C).
B) Sending cell wins: Epsin targets ubiquitinated Dl for Clathrin mediated endocytosis, applying force across the ligand receptor bridge that opens up the NRR (depicted as a spring) to uncover the S2 site for cleavage. Ectodomain shedding renders the remainder of the receptor subject to S3 cleavage, allowing the cytosolic domain to enter the nucleus and activate target genes. The available evidence suggests that Dl ubiquitination is normally induced by receptor binding (see Discussion).
C) Receiving cell wins: Ligand is internalized in its entirety by receptor-mediated ligand transendocytosis, possibly by engulfment of a patch of the sending cell surface in which the ligand is embedded, as depicted. Under normal conditions, receptor induced ubiquitination of ligand triggers Epsin-dependent S2 cleavage of the receptor before the receptor can transendocytose the ligand. However, manipulations or natural processes that compromise access of ligand to the Epsin/Clathin pathway, tip the competition in favor of the receiving cell, quenching the signal.