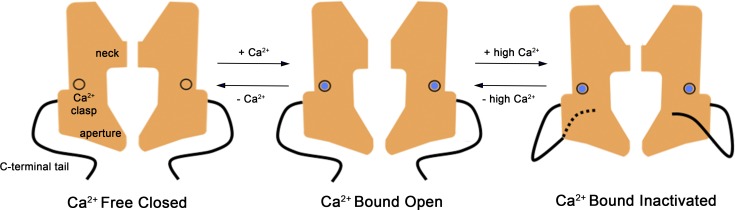
Figure 10.
Hypothesized mechanisms for activation and inactivation in bestrophin channels. In the Ca2+-free state of the channel the neck is sealed shut, and the channel is nonconductive. The binding of Ca2+ ions to the high-affinity Ca2+ clasps causes dilation of the neck, which now permits ions to flow through it. In both the Ca2+-free closed and Ca2+-bound open states, the inactivation peptides (one from each subunit) are not engaged with their receptors. In the presence of higher (e.g., μM) concentrations of Ca2+, the inactivation peptides bind to their receptor sites on the cytosolic surface of the channel (the dashed line indicates that this tail binds on the back surface). Inactivation peptide binding causes conformational changes in the channel that allosterically control the conformation of the neck of the pore, causing it to transition into a nonconductive conformation. The aperture does not have a role in activation or inactivation. Instead, the aperture functions as a size-selective filter that permits the passage of small ions and requires them to become at least partially dehydrated as they move through it and thereby gives rise to the lyotropic sequence of permeability among permeant anions (Vaisey et al., 2016).