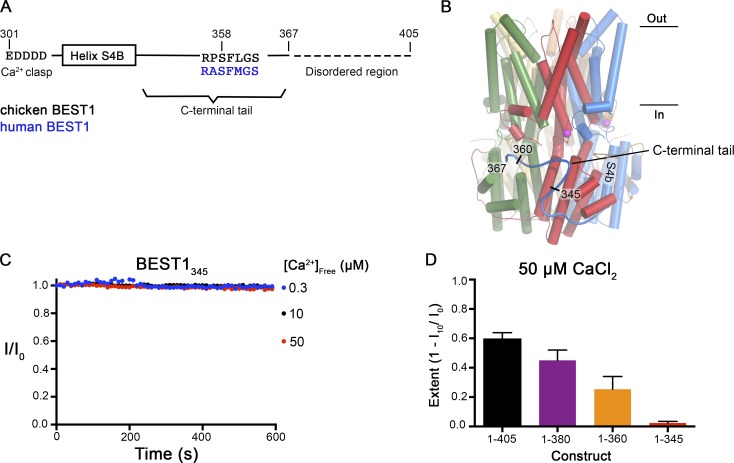
Figure 2.
Truncations of the C-terminal tail disrupt Ca2+-dependent inactivation of BEST1. (A) Schematic of the C-terminal region of BEST1, showing residues that contribute to the Ca clasp, helix S4b, and the C-terminal tail. The inhibitory sequence, contained within this tail, is annotated for both chicken and human BEST1. (B) Structure of BEST1WT highlighting the position of a C-terminal tail from one of the five identical subunits. The perspective is from within the membrane, with subunits colored individually, α-helices depicted as cylinders, Ca2+ ions shown as magenta spheres, and the approximate boundaries of the membrane indicated. Helix S4b and truncation points within the C-terminal are labeled. (C) Bilayer electrophysiology shows that BEST1345 does not undergo Ca2+-dependent inactivation. Experimental conditions are identical to those for Fig. 1, A and B. (D) The extent of inactivation in 50 µM Ca2+ is shown for different C-terminal truncations of BEST1. Experiments were performed as in C, and at least three independent experiments were performed for each construct to calculate SD.