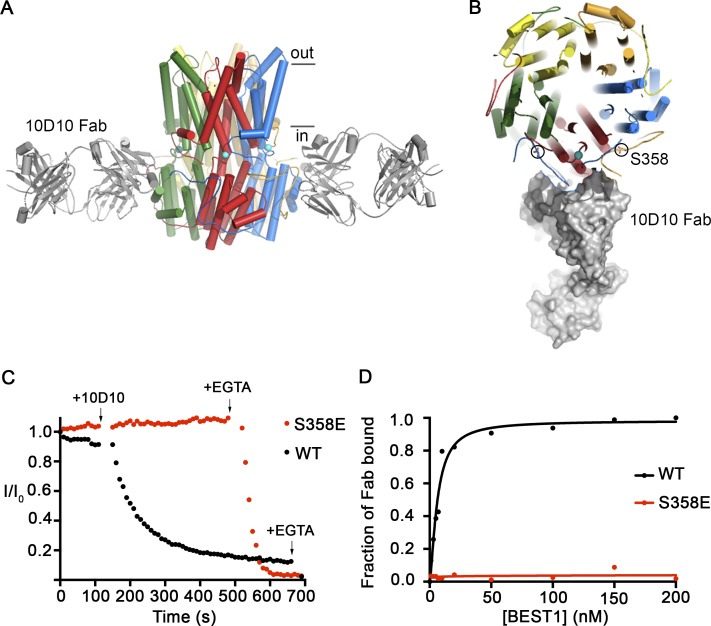
Figure 5.
The 10D10 Fab inhibits ionic currents of BEST1WT but not BEST1S358E. (A) Structure of the BEST1WT–10D10 complex, viewed from the side, showing approximate boundaries of the membrane. For clarity, two 10D10 Fabs are drawn (three are omitted). (B) A slice of the representation from A, viewed from the top, depicting one 10D10 Fab in surface representation (four are omitted for clarity). Two S358 residues from different subunits are circled. (C) The Ca2+-dependent inactivation of BEST1WT and BEST1S358E currents in 10 µM Ca2+ was recorded as described in Fig. 1 A. 200 nM 10D10 Fab was added to both cis and trans sides at the indicated time (arrow; for both BEST1WT and BEST1S358E), followed by the addition of 10 mM EGTA near the end of the experiment (arrow). (D) The relative binding of 10D10 Fab to BEST1WT and BEST1S358E was assayed by determining the amount of free 10D10 Fab as a function of the concentration of BEST1 (Materials and methods). The curves correspond to fits of the following: fraction of Fab bound = [BEST1]h/(Kdh + [BEST1]h), where Kd is the equilibrium dissociation constant, h is the Hill coefficient, and [BEST1] is the total concentration of BEST1. The derived parameters for BEST1WT are Kd ≤ 6 nM and h = 1.5, while BEST1S358E had no detectable binding to the 10D10 Fab.