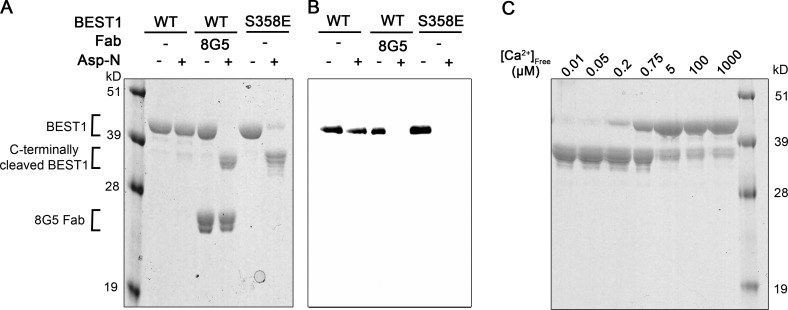
Figure 7.
Conditions or mutations that prevent inactivation result in increased susceptibility of the C-terminal tail to proteolytic cleavage. (A) Protease susceptibility of BEST1WT, BEST1WT-8G5, and BEST1S358E. Purified proteins (in detergent and [Ca2+]free ∼10 µM) were incubated with and without Asp-N endoproteinase for 1 h at room temperature and analyzed by SDS-PAGE with Coomassie staining. Bands corresponding to the 8G5 Fab and to uncleaved and C-terminally cleaved BEST1 are indicated. (B) Western blot of analogous SDS-PAGE gel from A using an antibody (YL1/2) that targets the C-terminal affinity tag used for purification of BEST1. (C) Protease susceptibility depends on [Ca2+]free. SDS-PAGE analysis of BEST1WT that had been treated with Asp-N protease for 1 h at room temperature using different [Ca2+]free.