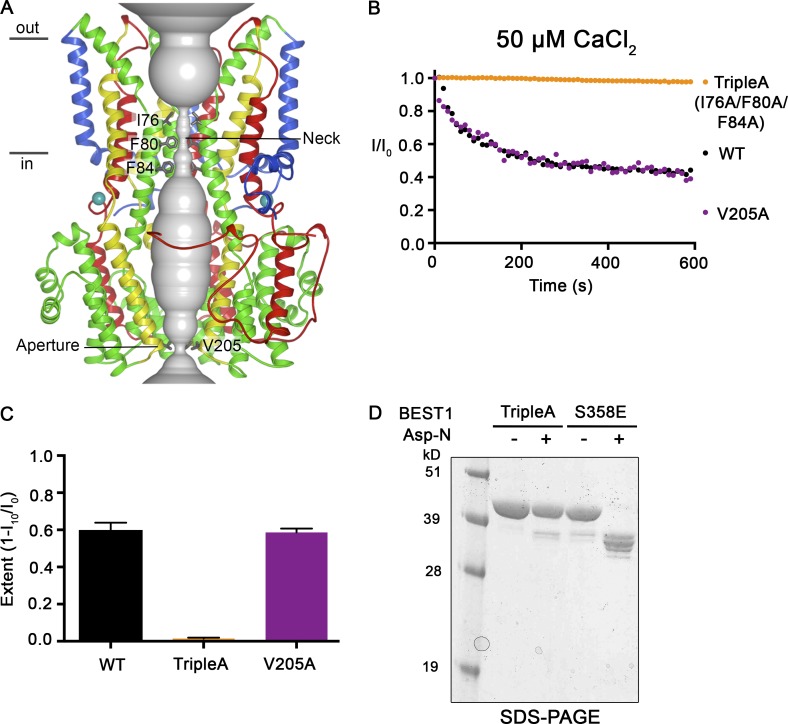
Figure 8.
Mutations within the neck, but not within the aperture, prevent Ca2+-dependent inactivation. (A) Structure of BEST1WT with ribbon representations shown for three subunits (two in the foreground are removed for clarity). The ion pore (gray tube) is depicted as a representation of the minimal radial distance from the central axis to the nearest van der Waals protein contact. Secondary structural elements are colored according to the four segments of the channel (S1, blue; S2, green; S3, yellow; and S4 and C-terminal tail, red) that contain corresponding transmembrane regions, and the Ca2+ ions are depicted as teal spheres. Approximate boundaries of a lipid membrane are indicated. (B) The Ca2+-dependent inactivation of BEST1WT, BEST1TripleA (I76A/F80A/F84A), and BEST1V205A, using 50 µM Ca2+, was recorded as described in Fig. 1 A. (C) The extent of inactivation in 50 µM Ca2+ is plotted for BEST1WT, BEST1TripleA, and BEST1V205A. Experiments were performed as in B, and at least three independent experiments were performed to calculate SD. (D) Like BEST1WT, BEST1TripleA is resistant to proteolytic cleavage by Asp-N in the presence of ∼10 µM [Ca2+]free. The BEST1TripleA and BEST1S358E (as a positive control for proteolytic cleavage) were incubated with or without Asp-N for 1 h at room temperature using conditions identical to those for Fig. 7 A. The samples were assayed by SDS-PAGE with Coomassie staining.