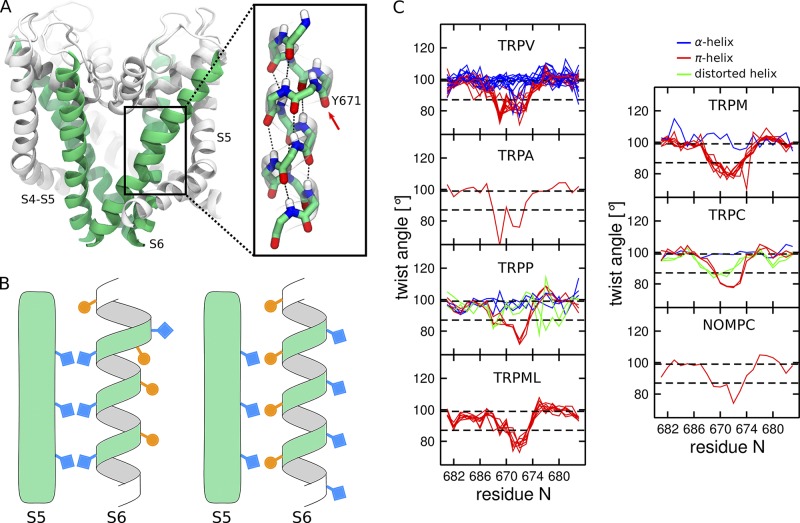
Figure 2.
π-helical segment in the S6 helix of TRP channels. (A) Cartoon representation of the TRPV1 pore domain (Cao et al., 2013b). The S6 helices are colored in green, and the rest of the pore domain in white. The inset shows the π-helical segment and the π-bulge located on the Y671 residue (highlighted with an arrow). The atoms are colored by element type: C in green, N in blue, O in red, and H in white. (B) Schematic representation of an α-helix (right) and an α-helix with a π-bulge (left). Note that the residues facing S5 in the α-helix are lining the pore when the π-bulge is introduced. (C) Per residue twist angle in the S6 helices of the available TRP structures estimated using HELANAL (Bansal et al., 2000). The structures with the π-bulge are shown in red (TRPV1, TRPV3, TRPV5, TRPV6, TRPA1, TRPM2, TRPM4, TRPM7, TRPC4, TRPP2, NOMPC, TRPML1, and TRPML3), and without the π-bulge in blue (TRPV2, TRPV3, TRPV4, TRPV5, TRPV6, TRPM8, TRPC3, and TRPP2). The structures in which S6 is distorted and cannot be assigned to either the α-helix or the α-helix with π-bulge are shown in green (TRPC3, TRPC6, and TRPP2). For the PDB codes of the structures, see Table 1. The twist angle is 99.4° and 86.6° for idealized α-helix and π-helix, respectively (highlighted by dashed black lines).