Figure 3.
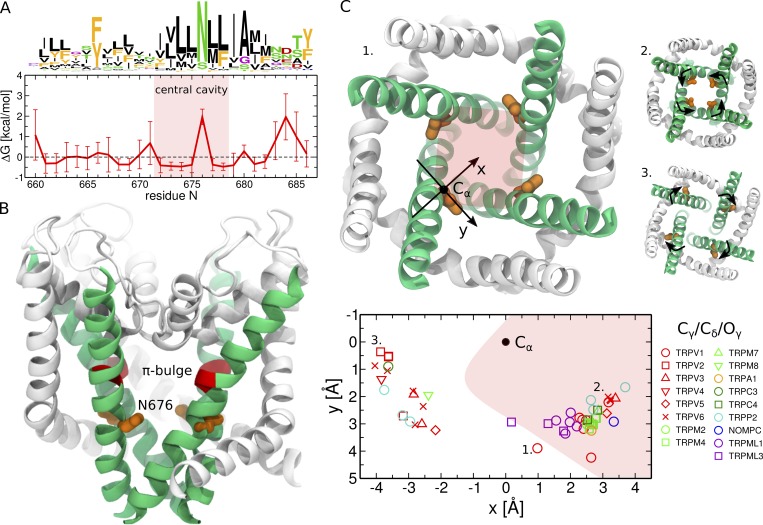
Conserved asparagine adopts a range of conformations from pointing toward the pore to pointing toward the S4–S5 linker. (A) Sequence logos and per-position hydrophobicity plot of the S6 helix across the TRP family (numbering based on the TRPV1 sequence; Palovcak et al., 2015). In the sequence logo, the height of each residue at a given position is proportional to its frequency at this position, and the height of the overall stack of residues decreases linearly with Shannon entropy (Crooks et al., 2004). Aromatic residues (F, W, Y) are shown in orange; hydrophobic not aromatic (A, C, I, L, M, V) in black; hydrophilic (N, Q, S, T) in green; positively (H, K, R) and negatively (D, E) charged in blue and red, respectively; and G and P in purple. Note that in a few cases, the conserved asparagine can be substituted by a serine (TRPML channels) or glutamine (TRPN-like channel NOMPC). In the hydrophobicity plot, positions with positive are hydrophilic, and those with negative are hydrophobic. The error bars represent SD. The red shaded area highlights the residues lining the central cavity (between Y671 and I679). (B) Cartoon representation of the TRPV1 pore domain (Cao et al., 2013b). The S6 helices are shown in green, and the rest of the pore domain in white. The π-bulge and the neighboring conserved asparagine are colored in red and orange, respectively. (C) Orientation of the conserved asparagine with respect to the pore in different TRP structures (Cao et al., 2013b; Liao et al., 2013; Barad et al., 2015; Paulsen et al., 2015; Gao et al., 2016; Huynh et al., 2016; Saotome et al., 2016; Shen et al., 2016; Zubcevic et al., 2016, 2018; Chen et al., 2017; Grieben et al., 2017; Guo et al., 2017; Hirschi et al., 2017; Jin et al., 2017; Schmiege et al., 2017; Singh et al., 2017, 2018a,b; Winkler et al., 2017; Zhou et al., 2017; Autzen et al., 2018; Deng et al., 2018; Duan et al., 2018a,b; Fan et al., 2018; Hughes et al., 2018b,a; Hulse et al., 2018; McGoldrick et al., 2018; Su et al., 2018a,b; Vinayagam et al., 2018; Yin et al., 2018; Zhang et al., 2018; Zheng et al., 2018). The side chain position in the plane perpendicular to the pore axis is shown: the Cα-atom is represented as a black dot and is located at (0,0), and the terminal Cγ-atom (Cδ and Oγ in NOMPC and TRPML channels, respectively) is shown as a colored symbol (a different one for each TRP channel). The x-axis is aligned with the vector connecting the Cα-atom and the center of the pore. The red shaded area highlights the pore region. The three insets show the pore domains of the TRPV1 capsaicin-bound structure (1), the TRPV6 open structure (2), and TRPV2 (3): the conserved asparagine points toward the S4–S5 linker in TRPV2, points toward the center of the pore in the TRPV6 open structure, and lies just outside the pore in the TRPV1 capsaicin-bound structure. In TRPV6 and TRPV2 (the two extreme cases), the black arrows highlight the direction of the asparagine rotation between the conformations inside and outside the pore.