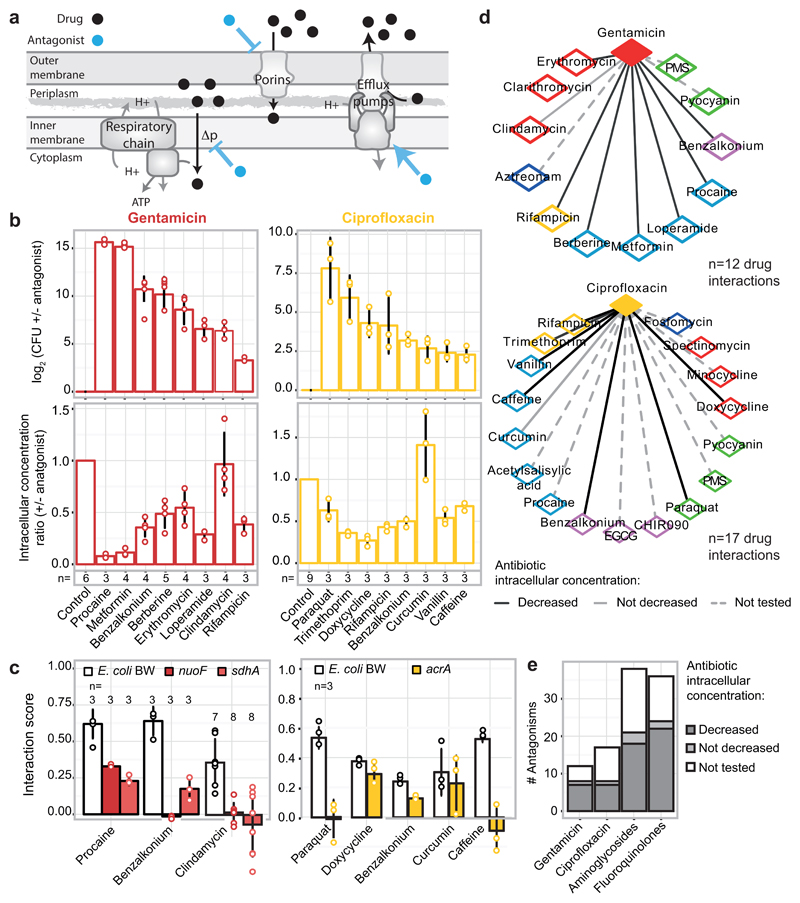
Extended Data Figure 8. Drug antagonisms are often due to decrease in intracellular drug concentrations.
a) Cartoon of possible MoAs for drug-drug interactions that function via modulation of the intracellular drug concentration. A drug (antagonist; blue) inhibits the uptake or promotes the efflux of another one (black), and thus decreases its intracellular concentration. b) Different antagonists (see methods for concentrations) of gentamicin (red – 5 µg/ml) and ciprofloxacin (gold – 2.5 µg/ml) identified in our screen for E. coli BW also rescue the killing effect of the two bactericidal drugs in the same strain or its parental MG1655 (top right and top left panel, respectively). With the exception of clindamycin (for gentamicin) and curcumin (for ciprofloxacin) all other antagonists decrease the intracellular concentration of their interacting drug (bottom panels) – gentamicin detected by using radiolabeled compound and ciprofloxacin with LC-MS/MS (Methods). The degree of rescue (upper panel) in many cases follows the decrease of intracellular concentration (lower panel), implying that most of these interactions depend at least partially on modulating the intracellular concentration of the antagonized drug. c) Antagonisms are resolved in E. coli BW mutants lacking key components controlling the intracellular concentration of the antagonized drug. Aminoglycosides depend on PMF-energized uptake and thus respiratory complexes 7,46; ciprofloxacin is effluxed by AcrAB-TolC 29,47. For gentamicin, most interactions are resolved when respiration is defected, even the one with clindamycin (not modulating intracellular gentamicin concentration- panel b) presumably because MoA and import of aminoglycosides are linked in a positive feedback loop 7,48. For ciprofloxacin, antagonisms with paraquat and caffeine are resolved in the ΔacrA mutant, implying that both compounds induce the AcrAB-TolC pump (known for paraquat). In contrast, interactions with curcumin, benzalkonium and doxycycline remain largely intact in the ΔacrA mutant. The first interaction is expected as curcumin does not modulate intracellular ciprofloxacin concentration (see panel b). In the other two cases, other component(s) besides AcrAB-TolC may be responsible for the altered ciprofloxacin import/export; for example, ciprofloxacin uses OmpF to enter the cell 49. Ciprofloxacin and gentamicin concentrations were adjusted in all strains according to MIC (70% and 100% MIC for ciprofloxacin and gentamicin, respectively; all drug concentrations are listed in Supplementary Table 6). Bliss interaction scores (ε) were calculated as in screen. Barplots and error bars in c & d represent the average and standard deviation, respectively, across n independent biological replicates. d) Gentamicin and ciprofloxacin antagonism networks for E. coli BW. Nodes represent drugs colored according to targeted cellular process (as ED Fig. 1a). Full and dashed edges represent antagonistic drug-drug interactions for which intracellular antibiotic concentration was and was not measured, respectively. Drug interactions that result in decreased intracellular concentration of the antagonized drug are represented by black edges. e) Quantification of antagonistic drug-drug interactions from the networks in (d). The bars for fluoroquinolones and aminoglycosides account for an extrapolation of antagonistic interactions to all other members of the two classes, assuming they behave the same as ciprofloxacin and gentamicin, respectively.