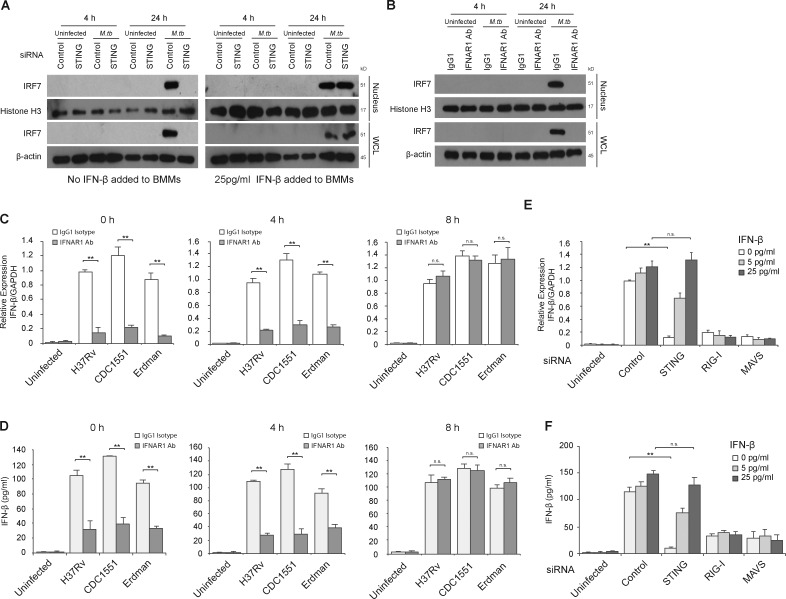
Figure 6.
Early STING-dependent production of IFN-β is required for activation of the RIG-I/MAVS/IRF7 signaling pathway. (A) Western blot for IRF7 in BMM WCLs or nuclear fractions (nucleus). BMMs were treated with control or STING siRNA and either left uninfected or infected for 4 or 24 h with WT M.tb CDC1551 strain at an MOI of 10. For one set of samples, BMMs were also treated with 25 pg/ml of IFN-β at the time of infection. Histone H3 and β-actin were used as loading controls for nuclear fraction and WCL, respectively. (B) WT BMMs were infected with WT M.tb CDC1551 strain in the presence of an IFN-β receptor blocking antibody (IFNAR1 Ab) or isotype control (IgG1). (C) IFNAR1 blocking Ab or control IgG1 were added at different times after infection (0, 4, and 8 h). IFN-β mRNA levels in BMMs were defined by qRT-PCR 24 h after M.tb infection (H37Rv, CDC1551, and Erdman) or in uninfected cells. (D) As described for C, except IFN-β protein levels in the culture supernatant were quantified by ELISA. (E) Use of qRT-PCR to define IFN-β mRNA expression levels in control, STING, RIG-I, or MAVS siRNA-treated BMMs following a 24-h infection with the WT M.tb CDC1551 strain or in uninfected cells. At the time of infection, recombinant mouse IFN-β was added to BMMs at a final concentration of 5 or 25 pg/ml or was left untreated. (F) Same as E, except IFN-β protein levels in culture supernatant were quantified by ELISA. For qRT-PCR, the mRNA expression levels of IFN-β were expressed as fold change relative to WT H37Rv-infected cells treated with isotype antibody (C) or infected BMMs treated with control siRNA, no IFN-β (E). Levels were normalized to GAPDH. Data are representative of at least three independent experiments. Data are mean ± SD of triplicate wells. n.s., not statistically significant; ** P < 0.01 by two-tailed Student’s t test.